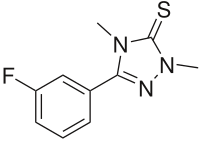
Chemical compound
Suritozole (MDL 26,479 ) is an investigational cognition enhancer . It acts as a partial inverse agonist at the benzodiazepine receptor site on the GABAA ion channel complex, but does not have either anxiogenic or convulsant effects, unlike other BZD inverse agonists such as DMCM .[1] depression and Alzheimer's disease ,[2]
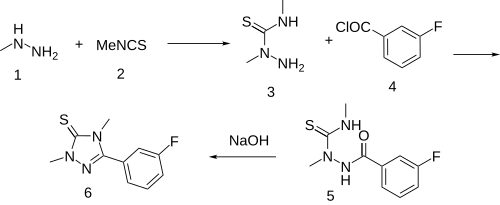
Synthesis [ edit ] Synthesis:[3] [4] [5] [6] [7] The reaction between monomethylhydrazine [60-34-4] (1 ) and methyl isothiocyanate (Trapex) [556-61-6] (2 ) gave 2,4-dimethylthiosemicarbazide [6621-75-6] (3 ). Amide formation with 3-fluorobenzoyl chloride [1711-07-5] (4 ) yielded 1-(3-fluorobenzoyl)-2,4-dimethylthiosemicarbazide [110623-52-4] (5 ). Cyclization to Suritozole (6 ).
See also [ edit ] References [ edit ]
^ Miller JA, Dudley MW, Kehne JH, Sorensen SM, Kane JM (September 1992). "MDL 26,479: a potential cognition enhancer with benzodiazepine inverse agonist-like properties" . Br. J. Pharmacol . 107 (1): 78–86. doi :10.1111/j.1476-5381.1992.tb14466.x . PMC 1907590 PMID 1330168 . ^ Robbins DK, Hutcheson SJ, Miller TD, Green VI, Bhargava VO, Weir SJ (May 1997). "Pharmacokinetics of MDL 26479, a novel benzodiazepine inverse agonist, in normal volunteers". Biopharm Drug Dispos . 18 (4): 325–34. doi :10.1002/(SICI)1099-081X(199705)18:4<325::AID-BDD21>3.0.CO;2-1 . PMID 9158880 . ^ Miller, JA; Dudley, MW; Kehne, JH; Sorensen, SM; Wenstrup, DL; Kane JM;. MDL 26,479 Drugs Fut 1992, 17, 1, 21.
^ Kane, John M.; Dudley, Mark W.; Sorensen, Stephen M.; Miller, Francis P. (1988). "2,4-Dihydro-3H-1,2,4-triazole-3-thiones as potential antidepressant agents". Journal of Medicinal Chemistry. 31 (6): 1253–1258. doi :10.1021/jm00401a031
^ Louks, David H.; Stolz-Dunn, Sandra K. (2007). "Kinetics for Scale-Up of a One-Pot Pathway to 5-(3-Fluorophenyl)-2,4-dihydro-2,4-dimethyl-3H-1,2,4-triazole-3-thione Using a Hybrid Model of Parallel and Consecutive Reactions". Organic Process Research & Development. 11 (5): 877–884. doi :10.1021/op700101g
^ US5856350 idem Christopher Robin Dalton, John Michael Kane, John Herr Kehne, WO 1996033177
^ US5723624 idem Sandra K. Stolz-Dunn, David H. Louks, Yolanda M. Puga, Christian T. Goralski, WO 1996001812
Ionotropic
GABAA Tooltip γ-Aminobutyric acid A receptor
Positive modulators (abridged; see here for a full list): α-EMTBL Alcohols (e.g., drinking alcohol , 2M2B )Anabolic steroids Avermectins (e.g., ivermectin )Barbiturates (e.g., phenobarbital )Benzodiazepines (e.g., diazepam )Bromide compounds (e.g., potassium bromide )Carbamates (e.g., meprobamate )Carbamazepine Chloralose Chlormezanone Clomethiazole Dihydroergolines (e.g., ergoloid (dihydroergotoxine) )Etazepine Etifoxine Fenamates (e.g., mefenamic acid )Flavonoids (e.g., apigenin , hispidulin )Fluoxetine Flupirtine Imidazoles (e.g., etomidate )Kava constituents (e.g., kavain )Lanthanum Loreclezole Monastrol Neuroactive steroids (e.g., allopregnanolone , cholesterol , THDOC )Niacin Niacinamide Nonbenzodiazepines (e.g., β-carbolines (e.g., abecarnil ), cyclopyrrolones (e.g., zopiclone ), imidazopyridines (e.g., zolpidem ), pyrazolopyrimidines (e.g., zaleplon ))Norfluoxetine Petrichloral Phenols (e.g., propofol )Phenytoin Piperidinediones (e.g., glutethimide )Propanidid Pyrazolopyridines (e.g., etazolate )Quinazolinones (e.g., methaqualone )Retigabine (ezogabine) ROD-188 Skullcap constituents (e.g., baicalin )Stiripentol Sulfonylalkanes (e.g., sulfonmethane (sulfonal) )Topiramate Valerian constituents (e.g., valerenic acid )Volatiles /gases (e.g., chloral hydrate , chloroform , diethyl ether , paraldehyde , sevoflurane )Negative modulators: 1,3M1B 3M2B 11-Ketoprogesterone 17-Phenylandrostenol α3IA α5IA (LS-193,268) β-CCB β-CCE β-CCM β-CCP β-EMGBL Anabolic steroids Amiloride Anisatin β-Lactams (e.g., penicillins , cephalosporins , carbapenems )Basmisanil Bemegride Bicyclic phosphates (TBPS , TBPO , IPTBO )BIDN Bilobalide Bupropion CHEB Chlorophenylsilatrane Cicutoxin Cloflubicyne Cyclothiazide DHEA DHEA-S Dieldrin (+)-DMBB DMCM DMPC EBOB Etbicyphat FG-7142 (ZK-31906) Fiproles (e.g., fipronil )Flavonoids (e.g., amentoflavone , oroxylin A )Flumazenil Fluoroquinolones (e.g., ciprofloxacin )Flurothyl Furosemide Golexanolone Iomazenil (123 I) IPTBO Isopregnanolone (sepranolone) L-655,708 Laudanosine Lindane MaxiPost Morphine Morphine-3-glucuronide MRK-016 Naloxone Naltrexone Nicardipine Nonsteroidal antiandrogens (e.g., apalutamide , bicalutamide , enzalutamide , flutamide , nilutamide )Oenanthotoxin Pentylenetetrazol (pentetrazol) Phenylsilatrane Picrotoxin (i.e., picrotin , picrotoxinin and dihydropicrotoxinin )Pregnenolone sulfate Propybicyphat PWZ-029 Radequinil Ro 15-4513 Ro 19-4603 RO4882224 RO4938581 Sarmazenil SCS Suritozole TB-21007 TBOB TBPS TCS-1105 Terbequinil TETS Thujone U-93631 Zinc ZK-93426 GABAA -ρ Tooltip γ-Aminobutyric acid A-rho receptor
Metabotropic
GABAB Tooltip γ-Aminobutyric acid B receptor