| Reformatsky reaction | |
|---|---|
| Named after | Sergey Reformatsky |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | reformatsky-reaction |
| RSC ontology ID | RXNO:0000036 |
The Reformatsky reaction (sometimes transliterated as Reformatskii reaction) is an organic reaction which condenses aldehydes or ketones with α-halo esters using metallic zinc to form β-hydroxy-esters:[1][2]

The organozinc reagent, also called a 'Reformatsky enolate', is prepared by treating an alpha-halo ester with zinc dust. Reformatsky enolates are less reactive than lithium enolates or Grignard reagents and hence nucleophilic addition to the ester group does not occur. The reaction was discovered by Sergey Nikolaevich Reformatsky.
Some reviews have been published.[3][4]
In addition[5] to aldehydes and ketones, it has also been shown that the Reformatsky enolate is able to react with acid chlorides,[6] imines,[7] nitriles (see Blaise reaction), and nitrones.[8] Moreover,[5] metals other than zinc have also been used, including magnesium,[9] iron,[10] cobalt,[11] nickel,[12] germanium,[13] cadmium,[14] indium,[15][16] barium,[17] and cerium.[18] Additionally,[5] metal salts are also applicable in place of metals, notably samarium(II) iodide,[19][20] chromium(II) chloride,[21] titanium(II) chloride,[22] cerium(III) halides such as cerium(III) iodide,[23] and titanocene(III) chloride.[24]
Structure of the reagent[edit]
The crystal structures of the THF complexes of the Reformatsky reagents tert-butyl bromozincacetate[25] and ethyl bromozincacetate[26] have been determined. Both form cyclic eight-membered dimers in the solid state, but differ in stereochemistry: the eight-membered ring in the ethyl derivative adopts a tub-shaped conformation and has cis bromo groups and cis THF ligands, whereas in the tert-butyl derivative, the ring is in a chair form and the bromo groups and THF ligands are trans. Note that, in contrast to lithium and boron enolates, which have the metal(loid)s exclusively bond to oxygen, the zinc enolate moiety in the Reformatsky reagents have zinc atoms that are simultaneously O- and C-bound and can therefore be described as "organometallic".
 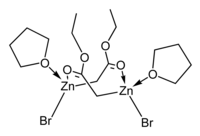 |
 
|
| ethyl bromozincacetate dimer | tert-butyl bromozincacetate dimer |
Reaction mechanism[edit]
Zinc metal is inserted into the carbon-halogen bond of the α-haloester by oxidative addition 1. This compound dimerizes and rearranges to form two zinc enolates 2. The oxygen on an aldehyde or ketone coordinates to the zinc to form the six-member chair like transition state 3. A rearrangement occurs in which zinc switches to the aldehyde or ketone oxygen and a carbon-carbon bond is formed 4. Acid workup 5,6 removes zinc to yield zinc(II) salts and a β-hydroxy-ester 7.[5]

Variations[edit]
In one variation of the Reformatsky reaction[27] an iodolactam is coupled with an aldehyde with triethylborane in toluene at -78 °C.
![Reformatsky reaction Danishefsky variation[27]](https://upload.wikimedia.org/wikipedia/commons/thumb/a/a7/Reformatskii_Danishefsky_2006.png/400px-Reformatskii_Danishefsky_2006.png)
See also[edit]
- Aldol reaction
- Blaise reaction
- Claisen condensation
- Example use in total synthesis: Mukaiyama Taxol total synthesis (B ring construction)
References[edit]
- ^ Reformatsky, S. (1887). "Neue Synthese zweiatomiger einbasischer Säuren aus den Ketonen". Berichte der Deutschen Chemischen Gesellschaft. 20 (1): 1210–1211. doi:10.1002/cber.188702001268.
- ^ Reformatsky, S. (1890). "Action of zinc and ethyl chloroacetate on ketones and aldehydes". J. Russ. Phys. Chem. Soc. 22: 44.
- ^ Shriner, R. L. (1942). "The Reformatsky Reaction". Organic Reactions. 1: 1–37. doi:10.1002/0471264180.or001.01. ISBN 9780471264187.
- ^ Rathke, M. W. (1975). "The Reformatsky Reaction". Organic Reactions. 22: 423–460. doi:10.1002/0471264180.or022.04. ISBN 0471264180.
- ^ a b c d Kurti, L.; Czako, B. Strategic Applications of Named Reactions in Organic Synthesis; Elsevier: Burlington, 2005.
- ^ Sato, Toshio; Itoh, Toshiyuki; Fujisawa, Tamotsu (1982). "Facile synthesis of β-ketoesters by a coupling reaction of the Reformatsky reagent with acyl chlorides catalyzed by a palladium complex". Chemistry Letters. 11 (10): 1559–1560. doi:10.1246/cl.1982.1559.
- ^ Gilman, Henry; Speeter, Merrill (1943). "The Reformatsky Reaction with Benzalaniline". Journal of the American Chemical Society. 65 (11): 2255–2256. doi:10.1021/ja01251a503.
- ^ Stamm, H.; Steudle, H. (1979). "Nitrone—XI Isoxazolidin-verbindungen—VIII : N-substituierte 5-isoxazolidinone durch reformatzky-reaktion mit nitronen". Tetrahedron. 35 (5): 647–650. doi:10.1016/0040-4020(79)87010-6.
- ^ Moriwake, Tosio (1966). "The Reformatsky Reaction. I. Condensation of Ketones and t-Butyl Bromoacetate by Magnesium". The Journal of Organic Chemistry. 31 (3): 983–985. doi:10.1021/jo01341a524.
- ^ Liu, Xuan-Yu; Li, Xiang-Rui; Zhang, Chen; Chu, Xue-Qiang; Rao, Weidong; Loh, Teck-Peng; Shen, Zhi-Liang (2019). "Iron(0)-Mediated Reformatsky Reaction for the Synthesis of β-Hydroxyl Carbonyl Compounds". Organic Letters. 21 (15): 5873–5878. doi:10.1021/acs.orglett.9b01999. PMID 31318222. S2CID 197541600.
- ^ Orsini, Fulvia; Pelizzoni, Francesca; Pulici, Maurizio; Vallarino, Lidia M. (1994). "A cobalt-phosphine complex as mediator in the formation of carbon-carbon bonds". The Journal of Organic Chemistry. 59 (1): 1–3. doi:10.1021/jo00080a001.
- ^ Inaba, Shin-ichi; Rieke, Reuben D. (1985). "Reformatsky type additions of haloacetonitriles to aldehydes mediated by metallic nickel". Tetrahedron Letters. 26 (2): 155–156. doi:10.1016/S0040-4039(00)61867-1.
- ^ Kagoshima, Hirotaka; Hashimoto, Yukihiko; Oguro, Dai; Saigo, Kazuhiko (1998). "An Activated Germanium Metal-Promoted, Highly Diastereoselective Reformatsky Reaction". The Journal of Organic Chemistry. 63 (3): 691–697. doi:10.1021/jo971672j. PMID 11672062.
- ^ Burkhardt, Elizabeth R.; Rieke, Reuben D. (1985). "The direct preparation of organocadmium compounds from highly reactive cadmium metal powders". The Journal of Organic Chemistry. 50 (3): 416–417. doi:10.1021/jo00203a036.
- ^ Chao, Li-Chung; Rieke, Reuben D. (1975). "Activated metals. IX. New reformatsky reagent involving activated indium for the preparation of β-hydroxy esters". The Journal of Organic Chemistry. 40 (15): 2253–2255. doi:10.1021/jo00903a031.
- ^ Araki, Shuki; Ito, Hirokazu; Butsugan, Yasuo (1988). "Synthesis of β-Hydroxyesters by Reformatsky Reaction Using Indium Metal". Synthetic Communications. 18 (4): 453–458. doi:10.1080/00397918808064009.
- ^ Yanagisawa, Akira; Takahashi, Hiroshi; Arai, Takayoshi (2004). "Reactive barium-promoted Reformatsky-type reaction of α-chloroketones with aldehydes". Chemical Communications (5): 580–581. doi:10.1039/B314752P. PMID 14973617.
- ^ Imamoto, Tsuneo; Kusumoto, Tetsuo; Tawarayama, Yoshinori; Sugiura, Yasushi; Mita, Takeshi; Hatanaka, Yasuo; Yokoyama, Masataka (1984). "Carbon-carbon bond-forming reactions using cerium metal or organocerium(III) reagents". The Journal of Organic Chemistry. 49 (21): 3904–3912. doi:10.1021/jo00195a006.
- ^ Tabuchi, Takanori; Kawamura, Kisa; Inanaga, Junji; Yamaguchi, Masaru (1986). "Preparation of medium- and large-ring lactones. SmI2-induced cyclization of ω-(α-bromoacyloxy) aldehydes". Tetrahedron Letters. 27 (33): 3889–3890. doi:10.1016/S0040-4039(00)83907-6.
- ^ Molander, Gary A.; Etter, Jeffrey B. (1987). "Lanthanides in organic synthesis. 8. 1.3-Asymmetric induction in intramolecular Reformatskii-type reactions promoted by samarium diiodide". Journal of the American Chemical Society. 109 (21): 6556–6558. doi:10.1021/ja00255a076.
- ^ Dubois, Jacques-Emile; Axiotis, Georges; Bertounesque, Emmanuel (1985). "Chromium (II) chloride : a new reagent for cross-aldol reactions". Tetrahedron Letters. 26 (36): 4371–4372. doi:10.1016/S0040-4039(00)98737-9.
- ^ Ishihara, Takashi; Yamanaka, Tohru; Ando, Teiichi (1984). "New low-valent titanium catalyzed reaction of chlorodifluoromethyl ketones leading to α,α-difluorinated β-hydroxy ketones". Chemistry Letters. 13 (7): 1165–1168. doi:10.1246/cl.1984.1165.
- ^ Fukuzawa, Shin-Ichi; Fujinami, Tatsuo; Sakai, Shizuyoshi (1985). "Carbon–carbon bond formation between α-halogenoketones and aldehydes promoted by cerium(III) iodide or cerium(III) chloride–sodium iodide". Journal of the Chemical Society, Chemical Communications (12): 777–778. doi:10.1039/C39850000777.
- ^ Parrish, J. D.; Shelton, Daniel R.; Little, R. Daniel (2003). "Titanocene(III)-Promoted Reformatsky Additions". Organic Letters. 5 (20): 3615–3617. doi:10.1021/ol035269c. PMID 14507186.
- ^ Dekker, J.; Budzelaar, P. H. M.; Boersma, J.; van der Kerk, G. J. M. & Spek, A. J. (1984). "The Nature of the Reformatsky Reagent. Crystal Structure of (BrZnCH2COO-t-Bu · THF)2". Organometallics. 9 (3): 1403–1407. doi:10.1021/om00087a015.
- ^ Miki, S.; Nakamoto, K.; Kawakami, J.; Handa, S.; Nuwa, S. (2008). "The First Isolation of Crystalline Ethyl Bromozincacetate, Typical Reformatsky Reagent: Crystal Structure and Convenient Preparation". Synthesis. 2008 (3): 409–412. doi:10.1055/s-2008-1032023.
- ^ a b Lambert, T. H.; Danishefsky, S. J. (2006). "Total Synthesis of UCS1025A". Journal of the American Chemical Society. 128 (2): 426–427. doi:10.1021/ja0574567. PMID 16402826.