 | |
| Clinical data | |
|---|---|
| Trade names | Vonedrine |
| Other names | N,β-Dimethylphenethylamine; N,β-Dimethylbenzeneethanamine; Phenylpropylmethylamine |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.257.912 |
| Chemical and physical data | |
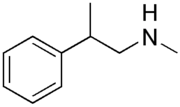
| Formula | C10H15N |
| Molar mass | 149.237 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Phenpromethamine (former brand name Vonedrine), also known as N,β-dimethylphenethylamine, is a sympathomimetic nasal decongestant of the phenethylamine group.[1][2][3][4] It was previously marketed as a nasal inhaler from 1943 through 1960 but is no longer available.[4][3][5] The medication is a stimulant and is banned by the World Anti-Doping Agency.[6][3] It has been detected in dietary supplements starting in the 2010s.[5][4]
See also[edit]
References[edit]
- ^ Weston AW, Ruddy AW, Suter CM (1943). "The Condensation of Unsaturated Amines with Aromatic Compounds. The Preparation of β-Substituted Phenethylamines". Journal of the American Chemical Society. 65 (4): 674–677. doi:10.1021/ja01244a049. ISSN 0002-7863.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 971–. ISBN 978-1-4757-2085-3.
- ^ a b c Docherty JR (June 2008). "Pharmacology of stimulants prohibited by the World Anti-Doping Agency (WADA)". British Journal of Pharmacology. 154 (3): 606–22. doi:10.1038/bjp.2008.124. PMC 2439527. PMID 18500382.
- ^ a b c Cohen PA, Travis JC, Vanhee C, Ohana D, Venhuis BJ (March 2021). "Nine prohibited stimulants found in sports and weight loss supplements: deterenol, phenpromethamine (Vonedrine), oxilofrine, octodrine, beta-methylphenylethylamine (BMPEA), 1,3-dimethylamylamine (1,3-DMAA), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylbutylamine (1,3-DMBA) and higenamine". Clinical Toxicology. 59 (11): 975–981. doi:10.1080/15563650.2021.1894333. PMID 33755516. S2CID 232338883.
- ^ a b Tsumura Y, Kiguchi A, Komatsuzaki S, Ieuji K (2019). "A novel method to distinguish β-methylphenylethylamines from isomeric α-methylphenylethylamines by liquid chromatography coupled to electrospray ionization mass spectrometry". Forensic Toxicology. 38 (2): 465–474. doi:10.1007/s11419-019-00511-z. ISSN 1860-8965. S2CID 212828763.
- ^ "The 2006 WADA Prohibited List: Summary of Revisions" (PDF). 2006 Canadian Centre for Ethics in Sport Advisory Notice. Archived from the original (PDF) on 11 April 2007.