| JADE1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | JADE1, PHF17, jade family PHD finger 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 610514 MGI: 1925835 HomoloGene: 18162 GeneCards: JADE1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
JADE1 is a protein that in humans is encoded by the JADE1 gene.[5][6][7][8]
Family[edit]
A small family of proteins named Gene for Apoptosis and Differentiation (JADE)[6] includes three members encoded by individual genes: Plant Homeo-domain-17 (PHF17, JADE1), PHF16 (JADE3), and PHF15 (JADE2). All JADE family proteins bear two notable mid molecule domains: the canonical Plant Homeo-domain (PHD) zinc finger and extended PHD-like zinc finger. JADE1 therefore is classified as a member of the PHD protein family. There are two known protein products of the PHF17 gene, the full length JADE1 (JADE1L) and its splice variant missing the C-terminal fragment also called short isoform (JADE1S).
Discovery[edit]
Nagase et al. cloned and sequenced 100 individual cDNAs from fetal brain cDNA library, including clone KIAA1807 which was designated PHF17.[9] The predicted 702-amino acid protein product of that clone was similar to the human zinc finger protein BR140 (BRPF1).[10] Based on sequence database analysis the study suggested that PHF17 may function in nucleic acid managing pathway.[9] Using yeast two hybrid pull down approach to search for new partners of protein product of the Von Hippel Lindau gene (pVHL) another study identified cDNA which matched to KIAA1807 clone.[11] The protein product of that cDNA was given name JADE1 (Jade-1, PHF17).[11] The deduced 509 amino acid long protein product of JADE1 cDNA was further confirmed as physical partner of pVHL.[11] In a genetic screen study searching for genes involved in embryogenesis, the mouse orthologue of JADE1 was identified.[6] That study, provided first characterization of the JADE1 gene and defined novel JADE family. The study yielded mice with knock out of JADE1 gene.
Jade1 transcripts in both humans and mice undergo alternative splicing and polyadenylation producing two major transcripts, the full length 6 kb mRNA and 3.6 kb mRNA.[6] Two resultant protein products of the JADE1 gene were designated JADE1S for the short (which is same as(3)) and JADE1L for the long isoform. Several minor transcripts are also detected. The database analysis revealed two additional JADE1 paralogues and members of JADE family, JADE2, and JADE3. JADE3 is identical to E9 protein identified in an earlier independent study which suggested role in apoptosis for PHF16/JADE3/E9 in breast cancer cells.[12]
JADE1 has been mapped to chromosome 4 (4q26-q27). JADE1 is conserved and its orthologues have been found or predicted in most every metazoan. Gene structure and sequences, variants, conservation, orthologues and paralogs, JADE1 phylogenetic tree and large scale screening of JADE1 tissue expression can be found in several extensive databases (https://www.genecards.org; http://useast.ensembl.org).
Structure[edit]
The full length JADE1 polypeptide bears one canonical and one extended PHD zinc finger domains.[13] Other domains include the N-terminal candidate PEST domain, Enhancer of polycomb-like domain and the C-terminal nuclear localization (NLS) signal[6][11] (prosite.expasy.org). JADE1 protein is a target for post translational modifications, including phosphorylation (Fig 1
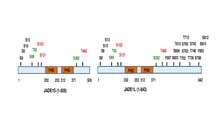
). Six amino acid residues were identified to be phosphorylated in cell cycle-dependent manner via Aurora A kinase pathway.[14][15] JADE1 is a target of phosphorylation by Casein kinase 2 (CK2).[16] In addition, multiple phosphorylation sites are found by high throughput screening approaches and in silico analysis. Summary schematic for JADE1L and JADE1S proteins phosphorylation sites with references are found in.[15]
Proteins bearing in tandem canonical and extended PHD fingers form a small subfamily within the large PHD protein family (www.genenames.org). Other proteins bearing tandem of PHD fingers and related to JADE1 include proteins that are components of chromatin binding and modifying complexes BRPF1, BRPF3, BRD1.[17] The crystal structure of JADE1 PHD domains has not been solved. Canonical PHD finger motif has signature C4HC3, represents relatively small, stable structure, and is distinct from the C3HC4 type RING finger. PHD domains are able to recognize and bind specific methylated lysine of histone H3, which defined these domains as epigenetic histone code readers.[18][19][20] Reviews describing structure and properties of PHD fingers in depth are available.[21][22][23][24]
Cellular function[edit]
JADE1 proteins are multifunctional and interact with several protein partners.
Histone acetylation[edit]
Function of JADE1 in histone acetylation and transcription activation which required the second extended PHD zinc finger was reported in 2004(16). JADE1 dramatically increases levels of acetylated histone H4 within chromatin, but not histone H3, a specificity characteristic to the MYST family HAT TIP60 and HBO1. TIP60 physically associates with JADE1 and augments JADE1 HAT function in live cells. TIP60 and JADE1 mutually stabilized each other. Transcriptional and HAT activities of JADE1 require PHD2. Results suggest the chromatin targeting role for JADE1 PHD2.[25] In addition, PHD2 of JADE1 binds the N-terminal tail of histone H3 within chromatin context irrespective of methylation status.[26]
Studies analyzing native complexes of INhibitor of Growth (ING) PHD finger family of proteins revealed that ING4 and ING5 proteins are associated with JADE1S and HAT HBO1,[27] while ING3 is associated with EPC1 (JADE1 homolog), TIP60 (HBO1 homolog) and several other partners. Both complexes also included a small Eaf6 protein. The biochemical and in silico analysis of complexes formed by HBO1 and TIP60 suggested common architecture and supported the role for JADE1 in bulk histone H4 acetylation. Characterization of JADE1 and HBO1 functional interactions show structural and functional similarities between the complexes(16, 19). Similarly to TIP60, JADE1 and HBO1 mutually stabilize each other.[28] JADE1 binds to and enables HBO1 to enhance global histone H4 acetylation, which requires intact PHD2 finger.[28] Similarly to HBO1, JADE1 is responsible for bulk histone H4 acetylation in cultured cells. H4K5, H4K12, and most likely H4K8 are targets of JADE1-dependent acetylation in cultured cells and in vivo.[14][26][29] Several potential transcription targets of JADE1 have been suggested from experiments using screening approaches.[26][30] According to screening genomic analysis done by ChIP-chip assay JADE1L complex is found mainly along the coding regions of many genes and JADE1L abundance correlates mostly with H3K36me3 histone mark. JADE1L over expression correlates with increased quantities of H4acK8 in the coding region of many genes.[26] The two PHD zinc fingers of JADE1 appear to bind preferentially non-methylated N-terminal peptide of histone.[26][30][31] JADE1 isoforms assemble at least two different complexes, JADE1L-HBO1-ING4/5 and JADE1S-HBO1 complex.[28] Due to the lack of the C-terminal fragment, JADE1S is incapable of binding ING4/5 partners.[28] A small less characterized protein Eaf6 is also another component of JADE1 complexes.[30]
Cell cycle[edit]
Acetylation of N-terminal fragments of bulk histone H4 has been known to correlate with DNA synthesis and cell division.[32][33][34][35][36] Several studies support cell cycle role for JADE1 linked to HBO1 pathway.[25][29] Both, JADE1 and HBO1 are independently required for the acetylation of bulk histone H4 in cultured cells.[25][27][28][29] The depletion of JADE1 proteins by siRNA results in 1) decreased levels of histone H4 bulk acetylation; 2) slower rates of DNA synthesis in cultured cells;[29] 3) decreased levels of the total and chromatin-bound HBO1;[28][29] 4) abrogation of chromatin recruitment of MCM7.[29] Agreeing with these results, JADE1L over-expression increases chromatin-bound MCM3 protein.[37] The effects of JADE1 depletion on DNA replication events are similar to those described originally for HBO1[38] and suggests adaptor role for JADE1 in HBO1-mediated cell cycle regulation.
JADE1 role in DNA damage has been suggested. A recently discovered non-coding RNA lncRNA-JADE regulates JADE1 expression and provides a functional link between the DNA damage response (DDR) and bulk histone H4 acetylation.[39] Results support role in DNA synthesis linked to histone H4 acetylation.[39] In cultured cells knock down of lncRNA-JADE increased cells sensitivity to DNA damaging drugs. In mice tumor xenograft model, the knock down of lncRNA-JADE inhibited xenograft mammary tumor growth. In a pilot human study, higher levels of lncRNA-JADE as well as JADE1 protein were detected in breast cancer tissues compared to normal tissues. Lastly, the higher levels of JADE1 protein inversely correlated with survival rates of patients with breast cancer. The study suggests that lncRNA-JADE might contribute to breast tumorigenesis, and that JADE1 protein mediates at least part of this effect.[39] JADE1 and cytokinesis. JADE1S negatively regulates cytokinesis of the epithelial cell cycle, a function specific to the small isoform.[14][15] First report that suggested JADE1 function in G2/M/G1 transition showed that during the late G2 phase, JADE1S undergoes phosphorylation linked to its dissociation from chromatin into the cytoplasm. Mass Spectral analysis identified that total of six individual amino acid residues are phosphorylated by a mitotic kinase.[14] Based on pharmacological analysis, JADE1 phosphorylation and compartmentalization is regulated by Aurora A and Aurora B pathways.[14][15] Other kinases have been reported and may play a role.[16][40] Upon completion of mitosis around telophase, the main pool of the JADE1S protein undergoes de-phosphorylation and re-associates with apparently condensing chromatin inside the reformed nuclei.[14] A discrete pool of JADE1S associates with the cleavage furrow and subsequently appears in the midbody of the cytokinetic bridge.[15] Only JADE1S, but not JADE1L or HBO1 was found in the midbody of the cells undergoing cytokinesis. The spatial regulation of JADE1S during the cell division suggested role in G2/M to G1 transition, which includes cytokinesis and final abscission.[15][41] Cytokinesis is the final step of cell cycle which controls fidelity of division of cellular content, including cytoplasm, membrane, and chromatin. Cytokinetic bridge is severed during the final abscission which occurs near the midbody and may take up to 2 hours. Cytokinesis and final abscission are tightly controlled by regulatory protein complexes and checkpoint proteins. The number of reports concerning cytokinesis control has been growing over the past decade.[42][43][44][45][46]
JADE1 role in cytokinesis was demonstrated by use of several functional assays and cell culture models.[15] DNA profiling by FACS showed that JADE1S depletion facilitated rates of G1-cells accumulation in synchronously dividing HeLa cells. The depletion of JADE1S protein in asynchronously dividing cells decreased the proportion of cytokinetic cells, and increased the proportion of multi-nuclear cells. The data demonstrated that JADE1 negatively controls cytokinesis, presumably by contributing to cytokinesis delay. JADE1 down-regulation increased number of multi-nuclear cells indicative of failed cytokinesis, while JADE1S moderate overexpression augmented the number of cytokinetic cells indicative of cytokinetic delay. Inhibition of Aurora B kinase by specific small molecule drugs resulted in the release of JADE1S-mediated cytokinetic delay and allowed progression of abscission. Since Aurora B is a key regulator of the NoCut, JADE1S is likely to regulate cytokinesis at the abscission checkpoint control.[15][41] JADE1S but not JADE1L or HBO1 was found in centrosomes of dividing cells throughout the cell cycle, and neither of these proteins was found in cilia. In contrast, another study reported JADE1 localization to the cilia and centrosome.[40] The study did not communicate on JADE1 isoform specificity.[40] Centrosomes are the cytoskeleton nucleation centers. Centrosome signaling contributes to the definition of cell shape, motility, orientation, polarity, division plane and to the fidelity of sister chromosome separation during mitosis and cytokinesis.[47][48]
pVHL[edit]
The first protein partner of JADE1S has been identified in 2002 in a study searching for new partners of the pVHL, which is a tumor suppressor.[11] A few follow up studies characterized binding and provided some insights on functional interactions of JADE1-pVHL.[49][50][51] The human pVHL is mutated in von Hippel–Lindau hereditary disease, and in majority of sporadic clear cell renal carcinomas.[52][53][54][55][56] Properties and function of pVHL have been investigated for many decades and extensive literature is available. One of the better known functions of pVHL is to mediate protein ubiquitination and proteosomal degradation. As a component of ubiquitin ligase E3 complex pVHL binds and targets several known factors, including HIF1a and HIF2a for ubiquitination.[55] Mechanism of HIF1a activation by hypoxia and role of pVHL in this pathway has been reported over a decade ago.[57] The VHL protein has been intensely studied and the link of naturally occurring mutations to cancers established. Other causative HIF-1a-independent pVHL pathways have been considered.[58] The pVHL-JADE1S physical interaction was identified by yeast-two hybrid screening analysis and was further confirmed biochemically. Co-transfection of pVHL increased JADE1S protein half-life and abundance, suggesting potential positive relationship.[11] Certain pVHL cancer-derived truncations but not point mutations diminished pVHL-JADE1 stabilization function, suggesting link to pVHL-associated cancers.[51] Molecular pathways and cellular significance of JADE1-pVHL interactions are not well understood. Single study describing JADE1S intrinsic ubiquitin-ligase activity and ubiquitination of beta-catenin has been reported in year 2008.[49] Based on that study a model has been proposed that pVHL regulates beta-catenin through JADE1, and PHD zinc fingers are required for this activity.
Apoptosis[edit]
JADE1S function in apoptosis has been proposed but the mechanisms remain elusive and results are hard to reconcile.[11][30][49][50][51] According to studies, JADE1 overexpression slows rates of cellular growth and induces cell cycle arrest protein p21. Several attempts to establish dependable cell lines stably expressing JADE1S protein have not been successful, presumably due to the negative cells self-selection. Contrary to that, another study shows that JADE1 downregulation decreased rates of DNA synthesis in synchronously dividing cells.[28][39] According to indirect immunofluorescence and microscopy analysis of cultured cells, cultured cells overload with JADE1 protein causes cell toxicity and side effects.[15] Cells undergo morphological changes that do not resemble apoptosis but suggest severely impaired cell cycle including dyeing cells with abnormal shapes and large, multi-lobular nuclei.[15] Based on JADE1S-mediated regulation of cell cycle other interpretations are considered: JADE1 overload might cause prolonged NoCut and stalled cytokinesis or severe cell cycle misbalance rather than direct transcription activation of apoptosis.[15]
Biological role[edit]
The biological role of JADE1 has not been elucidated. Limited number of publications addresses this question using mice models. The most comprehensive study which was published in 2003, identified mice orthologue of human JADE1, Jade1, and investigated Jade1 expression during mice embryogenesis.[6] Searching for developmentally regulated genes the authors used gene trap screen analysis and identified mouse Jade1 as gene strongly regulated during embryogenesis. Insertion of the vector into the third intron of the Jade1 gene lead to the production of a 47-amino-acid truncated protein. The gene trap insertional mutation resulted in Jade1-beta-galactosidase reporter fusion product and Jade1 null allele. While the homozygotes for the gene trap integration did not produce strong developmental phenotype, the fusion product revealed Jade1 gene spatial-temporal expression in mouse embryonic cells and tissues of developing embryo up to 15.5-d.p.c. In addition the study reports experimental and in silico comparative analysis of Jade1 mRNA transcripts, Jade1 gene structure and analysis of Jade1 protein orthologues from mouse human and zebra fish.[6] Jade1 expression was detected in extraembryonic ectoderm and trophoblast, which are placental components important for vasculogenesis, as well as in sites enriched with multipotent or tissue-specific progenitors, including neural progenitors(2). The dynamics of Jade1 reporter expression in these areas indicates the involvement in the determination and elongation of anterior posterior axis, an important point of the study).[6] The potential role for human JADE1 in the renewal of embryonic stem cell and embryonal carcinoma cell cultures was suggested in another screening study which showed that, in cultured stem cells activation of stem cell transcription factor OCT4 pathway upregulated JADE1 gene expression along with stem cell factors NANOG, PHC1, USP44 and SOX2.[59] Role of JADE1 in epithelial cell proliferation was addressed in a murine model of acute kidney injury and regeneration.[14][29] Expression patterns and dynamics of HBO1-JADE1S/L were examined in regenerating tubular epithelial cells.[29] Ischemia and reperfusion injury resulted in an initial decrease in JADE1S, JADE1L, and HBO1 protein levels, which returned to the baseline during renal recovery. Expression levels of HBO1 and JADE1S recovered as cell proliferation rate reached maximum, whereas JADE1L recovered after bulk proliferation had diminished. The temporal expression of JADE1 correlated with the acetylation of histone H4 (H4K5 and H4K12) but not that of histone H3 (H4K14), suggesting that the JADE1-HBO1 complex specifically marks H4 during epithelial cell proliferation. The results of the study implicate JADE1-HBO1 complex in acute kidney injury and suggest distinct roles for JADE1 isoforms during epithelial cell recovery.[29]
Disease associations[edit]
Role of JADE1 in human disease has not been elucidated. A recent study searched for novel submicroscopic genetic changes in myelofibrosis, which is a bone marrow cancer.[60] The study identified seven novel deletions and translocations in small cohort of patients with primary myelofibrosis. JADE1 and the adjacent gene called Sodium channel and clathrin linker 1 (SCLT1) were significantly modified. As a result of mutation, JADE1 gene has deletions of intron 5-6 and exons 6-11, which would produce JADE1 missing a large chunk of protein starting from the PHD zinc finger. The relevance to pathogenesis is under investigation. In a handful of pilot studies JADE1 expression was examined in colon cancers and renal carcinomas. The results in these studies do not always reconcile. The results of some studies are generated mostly from the histochemical analysis of tumor specimens using JADE1 antibody with uncharacterized specificities towards JADE1 in general, and JADE1S or JADE1L in particular.[61][62] Results of study using in silico microarray algorithm analysis shows, that PHF17 mRNA may play a role in the development of pancreatic cancer.[63] These promising lines of investigations require further controls and additional assessments.
Interactions[edit]
Several proteins interact with JADE1, including: pVHL,[11] TIP60,[25] HBO1, ING4, ING5,[28] β-catenin,[49] NPHP4.[40]
Notes[edit]
References[edit]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000077684 - Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000025764 - Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O (April 2001). "Prediction of the coding sequences of unidentified human genes. XX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Research. 8 (2): 85–95. doi:10.1093/dnares/8.2.85. PMID 11347906.
- ^ a b c d e f g h Tzouanacou E, Tweedie S, Wilson V (December 2003). "Identification of Jade1, a gene encoding a PHD zinc finger protein, in a gene trap mutagenesis screen for genes involved in anteroposterior axis development". Molecular and Cellular Biology. 23 (23): 8553–2. doi:10.1128/mcb.23.23.8553-8562.2003. PMC 262661. PMID 14612400.
- ^ Zhou MI, Wang H, Ross JJ, Kuzmin I, Xu C, Cohen HT (October 2002). "The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1". The Journal of Biological Chemistry. 277 (42): 39887–98. doi:10.1074/jbc.M205040200. PMID 12169691.
- ^ "Entrez Gene: PHF17 PHD finger protein 17".
- ^ a b Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O (April 2001). "Prediction of the coding sequences of unidentified human genes. XX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Research. 8 (2): 85–95. doi:10.1093/dnares/8.2.85. PMID 11347906.
- ^ Thompson KA, Wang B, Argraves WS, Giancotti FG, Schranck DP, Ruoslahti E (February 1994). "BR140, a novel zinc-finger protein with homology to the TAF250 subunit of TFIID". Biochemical and Biophysical Research Communications. 198 (3): 1143–52. doi:10.1006/bbrc.1994.1162. PMID 7906940.
- ^ a b c d e f g h Zhou MI, Wang H, Ross JJ, Kuzmin I, Xu C, Cohen HT (October 2002). "The von Hippel-Lindau tumor suppressor stabilizes novel plant homeodomain protein Jade-1". The Journal of Biological Chemistry. 277 (42): 39887–98. doi:10.1074/jbc.M205040200. PMID 12169691.
- ^ Szelei J, Soto AM, Geck P, Desronvil M, Prechtl NV, Weill BC, Sonnenschein C (March 2000). "Identification of human estrogen-inducible transcripts that potentially mediate the apoptotic response in breast cancer". The Journal of Steroid Biochemistry and Molecular Biology. 72 (3–4): 89–102. doi:10.1016/s0960-0760(00)00025-x. PMID 10775800. S2CID 25912630.
- ^ "Protein Jade-1 (Q6IE81)". InterPro.
- ^ a b c d e f g Siriwardana NS, Meyer R, Havasi A, Dominguez I, Panchenko MV (2014). "Cell cycle-dependent chromatin shuttling of HBO1-JADE1 histone acetyl transferase (HAT) complex". Cell Cycle. 13 (12): 1885–901. doi:10.4161/cc.28759. PMC 4111752. PMID 24739512.
- ^ a b c d e f g h i j k Siriwardana NS, Meyer RD, Panchenko MV (2015). "The novel function of JADE1S in cytokinesis of epithelial cells". Cell Cycle. 14 (17): 2821–34. doi:10.1080/15384101.2015.1068476. PMC 4612376. PMID 26151225.
- ^ a b Borgal L, Rinschen MM, Dafinger C, Hoff S, Reinert MJ, Lamkemeyer T, Lienkamp SS, Benzing T, Schermer B (September 2014). "Casein kinase 1 α phosphorylates the Wnt regulator Jade-1 and modulates its activity". The Journal of Biological Chemistry. 289 (38): 26344–56. doi:10.1074/jbc.M114.562165. PMC 4176241. PMID 25100726.
- ^ "JADE1 – Search – Homo sapiens – Ensembl genome browser 84".
- ^ Wysocka J, Swigut T, Xiao H, Milne TA, Kwon SY, Landry J, Kauer M, Tackett AJ, Chait BT, Badenhorst P, Wu C, Allis CD (July 2006). "A PHD finger of NURF couples histone H3 lysine 4 trimethylation with chromatin remodelling". Nature. 442 (7098): 86–90. Bibcode:2006Natur.442...86W. doi:10.1038/nature04815. PMID 16728976. S2CID 4389087.
- ^ Shi X, Hong T, Walter KL, Ewalt M, Michishita E, Hung T, Carney D, Peña P, Lan F, Kaadige MR, Lacoste N, Cayrou C, Davrazou F, Saha A, Cairns BR, Ayer DE, Kutateladze TG, Shi Y, Côté J, Chua KF, Gozani O (July 2006). "ING2 PHD domain links histone H3 lysine 4 methylation to active gene repression". Nature. 442 (7098): 96–9. Bibcode:2006Natur.442...96S. doi:10.1038/nature04835. PMC 3089773. PMID 16728974.
- ^ Taverna SD, Ilin S, Rogers RS, Tanny JC, Lavender H, Li H, Baker L, Boyle J, Blair LP, Chait BT, Patel DJ, Aitchison JD, Tackett AJ, Allis CD (December 2006). "Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs". Molecular Cell. 24 (5): 785–96. doi:10.1016/j.molcel.2006.10.026. PMC 4690528. PMID 17157260.
- ^ Sanchez R, Zhou MM (July 2011). "The PHD finger: a versatile epigenome reader". Trends in Biochemical Sciences. 36 (7): 364–72. doi:10.1016/j.tibs.2011.03.005. PMC 3130114. PMID 21514168.
- ^ Kwan AH, Gell DA, Verger A, Crossley M, Matthews JM, Mackay JP (July 2003). "Engineering a protein scaffold from a PHD finger". Structure. 11 (7): 803–13. doi:10.1016/s0969-2126(03)00122-9. PMID 12842043.
- ^ Mansfield RE, Musselman CA, Kwan AH, Oliver SS, Garske AL, Davrazou F, Denu JM, Kutateladze TG, Mackay JP (April 2011). "Plant homeodomain (PHD) fingers of CHD4 are histone H3-binding modules with preference for unmodified H3K4 and methylated H3K9". The Journal of Biological Chemistry. 286 (13): 11779–91. doi:10.1074/jbc.M110.208207. PMC 3064229. PMID 21278251.
- ^ Matthews JM, Bhati M, Lehtomaki E, Mansfield RE, Cubeddu L, Mackay JP (2009). "It takes two to tango: the structure and function of LIM, RING, PHD and MYND domains". Current Pharmaceutical Design. 15 (31): 3681–96. doi:10.2174/138161209789271861. PMID 19925420.
- ^ a b c d Panchenko MV, Zhou MI, Cohen HT (December 2004). "von Hippel-Lindau partner Jade-1 is a transcriptional co-activator associated with histone acetyltransferase activity". The Journal of Biological Chemistry. 279 (53): 56032–41. doi:10.1074/jbc.M410487200. PMID 15502158.
- ^ a b c d e Saksouk N, Avvakumov N, Champagne KS, Hung T, Doyon Y, Cayrou C, Paquet E, Ullah M, Landry AJ, Côté V, Yang XJ, Gozani O, Kutateladze TG, Côté J (January 2009). "HBO1 HAT complexes target chromatin throughout gene coding regions via multiple PHD finger interactions with histone H3 tail". Molecular Cell. 33 (2): 257–65. doi:10.1016/j.molcel.2009.01.007. PMC 2677731. PMID 19187766.
- ^ a b Doyon Y, Cayrou C, Ullah M, Landry AJ, Côté V, Selleck W, Lane WS, Tan S, Yang XJ, Côté J (January 2006). "ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation". Molecular Cell. 21 (1): 51–64. doi:10.1016/j.molcel.2005.12.007. PMID 16387653.
- ^ a b c d e f g h Foy RL, Song IY, Chitalia VC, Cohen HT, Saksouk N, Cayrou C, Vaziri C, Côté J, Panchenko MV (October 2008). "Role of Jade-1 in the histone acetyltransferase (HAT) HBO1 complex". The Journal of Biological Chemistry. 283 (43): 28817–26. doi:10.1074/jbc.M801407200. PMC 2570895. PMID 18684714.
- ^ a b c d e f g h i Havasi A, Haegele JA, Gall JM, Blackmon S, Ichimura T, Bonegio RG, Panchenko MV (January 2013). "Histone acetyl transferase (HAT) HBO1 and JADE1 in epithelial cell regeneration". The American Journal of Pathology. 182 (1): 152–62. doi:10.1016/j.ajpath.2012.09.017. PMC 3532714. PMID 23159946.
- ^ a b c d Avvakumov N, Lalonde ME, Saksouk N, Paquet E, Glass KC, Landry AJ, Doyon Y, Cayrou C, Robitaille GA, Richard DE, Yang XJ, Kutateladze TG, Côté J (February 2012). "Conserved molecular interactions within the HBO1 acetyltransferase complexes regulate cell proliferation". Molecular and Cellular Biology. 32 (3): 689–703. doi:10.1128/MCB.06455-11. PMC 3266594. PMID 22144582.
- ^ Lalonde ME, Avvakumov N, Glass KC, Joncas FH, Saksouk N, Holliday M, Paquet E, Yan K, Tong Q, Klein BJ, Tan S, Yang XJ, Kutateladze TG, Côté J (September 2013). "Exchange of associated factors directs a switch in HBO1 acetyltransferase histone tail specificity". Genes & Development. 27 (18): 2009–24. doi:10.1101/gad.223396.113. PMC 3792477. PMID 24065767.
- ^ Megee PC, Morgan BA, Smith MM (July 1995). "Histone H4 and the maintenance of genome integrity". Genes & Development. 9 (14): 1716–27. doi:10.1101/gad.9.14.1716. PMID 7622036.
- ^ Maki N, Tsonis PA, Agata K (16 September 2010). "Changes in global histone modifications during dedifferentiation in newt lens regeneration". Molecular Vision. 16: 1893–7. PMC 2956703. PMID 21031136.
- ^ Jasencakova Z, Meister A, Walter J, Turner BM, Schubert I (November 2000). "Histone H4 acetylation of euchromatin and heterochromatin is cell cycle dependent and correlated with replication rather than with transcription". The Plant Cell. 12 (11): 2087–100. doi:10.1105/tpc.12.11.2087. PMC 150160. PMID 11090211.
- ^ Clarke AS, Lowell JE, Jacobson SJ, Pillus L (April 1999). "Esa1p is an essential histone acetyltransferase required for cell cycle progression". Molecular and Cellular Biology. 19 (4): 2515–26. doi:10.1128/mcb.19.4.2515. PMC 84044. PMID 10082517.
- ^ Choy JS, Tobe BT, Huh JH, Kron SJ (November 2001). "Yng2p-dependent NuA4 histone H4 acetylation activity is required for mitotic and meiotic progression". The Journal of Biological Chemistry. 276 (47): 43653–62. doi:10.1074/jbc.M102531200. PMID 11544250.
- ^ Miotto B, Struhl K (January 2010). "HBO1 histone acetylase activity is essential for DNA replication licensing and inhibited by Geminin". Molecular Cell. 37 (1): 57–66. doi:10.1016/j.molcel.2009.12.012. PMC 2818871. PMID 20129055.
- ^ Iizuka M, Matsui T, Takisawa H, Smith MM (February 2006). "Regulation of replication licensing by acetyltransferase Hbo1". Molecular and Cellular Biology. 26 (3): 1098–108. doi:10.1128/MCB.26.3.1098-1108.2006. PMC 1347032. PMID 16428461.
- ^ a b c d Wan G, Hu X, Liu Y, Han C, Sood AK, Calin GA, Zhang X, Lu X (October 2013). "A novel non-coding RNA lncRNA-JADE connects DNA damage signalling to histone H4 acetylation". The EMBO Journal. 32 (21): 2833–47. doi:10.1038/emboj.2013.221. PMC 3817469. PMID 24097061.
- ^ a b c d Borgal L, Habbig S, Hatzold J, Liebau MC, Dafinger C, Sacarea I, Hammerschmidt M, Benzing T, Schermer B (July 2012). "The ciliary protein nephrocystin-4 translocates the canonical Wnt regulator Jade-1 to the nucleus to negatively regulate β-catenin signaling". The Journal of Biological Chemistry. 287 (30): 25370–80. doi:10.1074/jbc.M112.385658. PMC 3408186. PMID 22654112.
- ^ a b Prekeris R (2015). "Cut or NoCut: the role of JADE1S in regulating abscission checkpoint". Cell Cycle. 14 (20): 3219. doi:10.1080/15384101.2015.1089074. PMC 4825624. PMID 26327571.
- ^ Agromayor M, Martin-Serrano J (September 2013). "Knowing when to cut and run: mechanisms that control cytokinetic abscission". Trends in Cell Biology. 23 (9): 433–41. doi:10.1016/j.tcb.2013.04.006. PMID 23706391.
- ^ Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J (March 2011). "Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission". Proceedings of the National Academy of Sciences of the United States of America. 108 (12): 4846–51. Bibcode:2011PNAS..108.4846E. doi:10.1073/pnas.1102714108. PMC 3064317. PMID 21383202.
- ^ Fabbro M, Zhou BB, Takahashi M, Sarcevic B, Lal P, Graham ME, Gabrielli BG, Robinson PJ, Nigg EA, Ono Y, Khanna KK (October 2005). "Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis". Developmental Cell. 9 (4): 477–88. doi:10.1016/j.devcel.2005.09.003. PMID 16198290.
- ^ Green RA, Paluch E, Oegema K (2012). "Cytokinesis in animal cells". Annual Review of Cell and Developmental Biology. 28: 29–58. doi:10.1146/annurev-cellbio-101011-155718. PMID 22804577.
- ^ Hu CK, Coughlin M, Mitchison TJ (March 2012). "Midbody assembly and its regulation during cytokinesis". Molecular Biology of the Cell. 23 (6): 1024–34. doi:10.1091/mbc.E11-08-0721. PMC 3302730. PMID 22278743.
- ^ Nigg EA, Stearns T (October 2011). "The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries". Nature Cell Biology. 13 (10): 1154–60. doi:10.1038/ncb2345. PMC 3947860. PMID 21968988.
- ^ Sluder G, Khodjakov A (December 2010). "Centriole duplication: analogue control in a digital age". Cell Biology International. 34 (12): 1239–45. doi:10.1042/CBI20100612. PMC 3051170. PMID 21067522.
- ^ a b c d Chitalia VC, Foy RL, Bachschmid MM, Zeng L, Panchenko MV, Zhou MI, Bharti A, Seldin DC, Lecker SH, Dominguez I, Cohen HT (October 2008). "Jade-1 inhibits Wnt signalling by ubiquitylating beta-catenin and mediates Wnt pathway inhibition by pVHL". Nature Cell Biology. 10 (10): 1208–16. doi:10.1038/ncb1781. PMC 2830866. PMID 18806787.
- ^ a b Zhou MI, Foy RL, Chitalia VC, Zhao J, Panchenko MV, Wang H, Cohen HT (August 2005). "Jade-1, a candidate renal tumor suppressor that promotes apoptosis". Proceedings of the National Academy of Sciences of the United States of America. 102 (31): 11035–40. Bibcode:2005PNAS..10211035Z. doi:10.1073/pnas.0500757102. PMC 1182408. PMID 16046545.
- ^ a b c Zhou MI, Wang H, Foy RL, Ross JJ, Cohen HT (February 2004). "Tumor suppressor von Hippel-Lindau (VHL) stabilization of Jade-1 protein occurs through plant homeodomains and is VHL mutation dependent". Cancer Research. 64 (4): 1278–86. doi:10.1158/0008-5472.can-03-0884. PMID 14973063.
- ^ Crossey PA, Richards FM, Foster K, Green JS, Prowse A, Latif F, Lerman MI, Zbar B, Affara NA, Ferguson-Smith MA (August 1994). "Identification of intragenic mutations in the von Hippel-Lindau disease tumour suppressor gene and correlation with disease phenotype". Human Molecular Genetics. 3 (8): 1303–8. doi:10.1093/hmg/3.8.1303. PMID 7987306.
- ^ Foster K, Prowse A, van den Berg A, Fleming S, Hulsbeek MM, Crossey PA, Richards FM, Cairns P, Affara NA, Ferguson-Smith MA (December 1994). "Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma". Human Molecular Genetics. 3 (12): 2169–73. doi:10.1093/hmg/3.12.2169. PMID 7881415.
- ^ Duan DR, Humphrey JS, Chen DY, Weng Y, Sukegawa J, Lee S, Gnarra JR, Linehan WM, Klausner RD (July 1995). "Characterization of the VHL tumor suppressor gene product: localization, complex formation, and the effect of natural inactivating mutations". Proceedings of the National Academy of Sciences of the United States of America. 92 (14): 6459–63. Bibcode:1995PNAS...92.6459D. doi:10.1073/pnas.92.14.6459. PMC 41537. PMID 7604013.
- ^ a b Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ (May 1999). "The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis". Nature. 399 (6733): 271–5. Bibcode:1999Natur.399..271M. doi:10.1038/20459. PMID 10353251. S2CID 4427694.
- ^ Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L (May 1993). "Identification of the von Hippel-Lindau disease tumor suppressor gene". Science. 260 (5112): 1317–20. Bibcode:1993Sci...260.1317L. doi:10.1126/science.8493574. PMID 8493574.
- ^ Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, von Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ (April 2001). "Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation". Science. 292 (5516): 468–72. Bibcode:2001Sci...292..468J. doi:10.1126/science.1059796. PMID 11292861. S2CID 20914281.
- ^ Gossage L, Eisen T, Maher ER (January 2015). "VHL, the story of a tumour suppressor gene". Nature Reviews. Cancer. 15 (1): 55–64. doi:10.1038/nrc3844. PMID 25533676. S2CID 19312746.
- ^ Jung M, Peterson H, Chavez L, Kahlem P, Lehrach H, Vilo J, Adjaye J (21 May 2010). "A data integration approach to mapping OCT4 gene regulatory networks operative in embryonic stem cells and embryonal carcinoma cells". PLOS ONE. 5 (5): e10709. Bibcode:2010PLoSO...510709J. doi:10.1371/journal.pone.0010709. PMC 2873957. PMID 20505756.
- ^ Lasho T, Johnson SH, Smith DI, Crispino JD, Pardanani A, Vasmatzis G, Tefferi A (September 2013). "Identification of submicroscopic genetic changes and precise breakpoint mapping in myelofibrosis using high resolution mate-pair sequencing". American Journal of Hematology. 88 (9): 741–6. doi:10.1002/ajh.23495. PMID 23733509. S2CID 5232311.
- ^ Lian X, Duan X, Wu X, Li C, Chen S, Wang S, Cai Y, Weng Z (August 2012). "Expression and clinical significance of von Hippel-Lindau downstream genes: Jade-1 and β-catenin related to renal cell carcinoma". Urology. 80 (2): 485.e7–13. doi:10.1016/j.urology.2012.02.024. PMID 22516360.
- ^ Lim SR, Gooi BH, Singh M, Gam LH (November 2011). "Analysis of differentially expressed proteins in colorectal cancer using hydroxyapatite column and SDS-PAGE". Applied Biochemistry and Biotechnology. 165 (5–6): 1211–24. doi:10.1007/s12010-011-9339-3. PMID 21863284. S2CID 13272576.
- ^ Liu PF, Jiang WH, Han YT, He LF, Zhang HL, Ren H (28 August 2015). "Integrated microRNA-mRNA analysis of pancreatic ductal adenocarcinoma". Genetics and Molecular Research. 14 (3): 10288–97. doi:10.4238/2015.August.28.14. PMID 26345967.
Further reading[edit]
- Tzouanacou E, Tweedie S, Wilson V (December 2003). "Identification of Jade1, a gene encoding a PHD zinc finger protein, in a gene trap mutagenesis screen for genes involved in anteroposterior axis development". Molecular and Cellular Biology. 23 (23): 8553–2. doi:10.1128/MCB.23.23.8553-8562.2003. PMC 262661. PMID 14612400.
- Panchenko MV, Zhou MI, Cohen HT (December 2004). "von Hippel-Lindau partner Jade-1 is a transcriptional co-activator associated with histone acetyltransferase activity". The Journal of Biological Chemistry. 279 (53): 56032–41. doi:10.1074/jbc.M410487200. PMID 15502158.
- Zhou MI, Foy RL, Chitalia VC, Zhao J, Panchenko MV, Wang H, Cohen HT (August 2005). "Jade-1, a candidate renal tumor suppressor that promotes apoptosis". Proceedings of the National Academy of Sciences of the United States of America. 102 (31): 11035–40. Bibcode:2005PNAS..10211035Z. doi:10.1073/pnas.0500757102. PMC 1182408. PMID 16046545.
- Doyon Y, Cayrou C, Ullah M, Landry AJ, Côté V, Selleck W, Lane WS, Tan S, Yang XJ, Côté J (January 2006). "ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation". Molecular Cell. 21 (1): 51–64. doi:10.1016/j.molcel.2005.12.007. PMID 16387653.
- Lim J, Hao T, Shaw C, Patel AJ, Szabó G, Rual JF, Fisk CJ, Li N, Smolyar A, Hill DE, Barabási AL, Vidal M, Zoghbi HY (May 2006). "A protein-protein interaction network for human inherited ataxias and disorders of Purkinje cell degeneration". Cell. 125 (4): 801–14. doi:10.1016/j.cell.2006.03.032. PMID 16713569. S2CID 13709685.
- Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (November 2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983. S2CID 7827573.
External links[edit]
- PHF17+protein,+human at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.