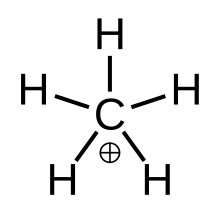
 "True" methanium, the metastable transitional state [CH5]+
| |
 Fluxional methanium, [CH3(H2)]+
| |
| Names | |
|---|---|
| IUPAC name
Methanium
| |
| Other names
carbonium (discouraged due to multiple definitions)[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| CH+5 | |
| Molar mass | 17.051 g·mol−1 |
| Conjugate base | Methane |
| Structure | |
| trigonal bipyramidal | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In chemistry, methanium is a complex positive ion with formula [CH5]+ (metastable transitional form, a carbon atom covalently bonded to five hydrogen atoms) or [CH3(H2)]+ (fluxional form, namely a molecule with one carbon atom covalently bonded to three hydrogen atoms and one dihydrogen molecule), bearing a +1 electric charge. It is a superacid and one of the onium ions, indeed the simplest carbonium ion.
It is highly unstable and highly reactive even upon having a complete octet, thus granting its superacidic properties.
Methanium can be produced in the laboratory as a rarefied gas or as a dilute species in superacids. It was prepared for the first time in 1950 and published in 1952 by Victor Talrose and his assistant Anna Konstantinovna Lyubimova.[2][3] It occurs as an intermediate species in chemical reactions.
The methanium ion is named after methane (CH4), by analogy with the derivation of ammonium ion (NH+4) from ammonia (NH3).
Structure[edit]
Fluxional methanium can be visualised as a CH+3 carbenium ion with a molecule of hydrogen interacting with the empty orbital in a 3-center-2-electron bond. The bonding electron pair in the H2 molecule is shared between the two hydrogen and one carbon atoms making up the 3-center-2-electron bond.[4]
The two hydrogen atoms in the H2 molecule can continuously exchange positions with the three hydrogen atoms in the CH+3 ion (a conformation change called pseudorotation, specifically the Berry mechanism). The methanium ion is therefore considered a fluxional molecule. The energy barrier for the exchange is quite low and occurs even at very low temperatures.[5][6]
Infrared spectroscopy has been used to obtain information about the different conformations of the methanium ion.[7][8][9] The IR spectrum of plain methane has two C-H bands from symmetric and asymmetric stretching at around 3000 cm−1 and two bands around 1400 cm−1 from symmetrical and asymmetric bending vibrations. In the spectrum of CH+5 three asymmetric stretching vibrations are present around 2800–3000 cm−1, a rocking vibration at 1300 cm−1, and a bending vibration at 1100 1300 cm−1.
Preparation[edit]
Methanium can be prepared from methane by the action of very strong acids, such as fluoroantimonic acid (antimony pentafluoride SbF5 in hydrogen fluoride HF).[10]
At about 270 Pa of pressure and ambient temperature, the methane ion CH+4 will react with neutral methane to yield methanium and a methyl radical:[11]
- CH+4 + CH4 → CH+5 + CH3•
The methanium ion can also be made in the gas phase via the reaction of methane and an H+ ion (i.e. a proton).[citation needed]
- CH4 + H+(g) → CH+5
Stability and reactions[edit]
The cations obtained by reaction of methane with SbF5 + HF, including methanium, are stabilized by interactions with the HF molecules.
At low pressures (around 1 mmHg) and ambient temperatures, methanium is unreactive towards neutral methane.[11]
Further reading[edit]
- D. W. Boo; Z. F. Liu; A. G. Suits; J. S. Tse; Y. T. Lee (1995). "Dynamics of Carbonium Ions Solvated by Molecular Hydrogen: CH5+(H2)n (n= 1, 2, 3)". Science. 269 (5220): 57–9. Bibcode:1995Sci...269...57B. doi:10.1126/science.269.5220.57. PMID 17787703. S2CID 2100896.
- Kramer, G. M. (1999). "CH5+ Stability and Mass Spectrometry". Science. 286 (5442): 1051a–1051. doi:10.1126/science.286.5442.1051a. ISSN 0036-8075.
- Marx, D. (1999). "MOLECULAR SPECTROSCOPY:CH5+: The Cheshire Cat Smiles". Science. 284 (5411): 59–61. Bibcode:1999Sci...284...59.. doi:10.1126/science.284.5411.59. ISSN 0036-8075. S2CID 94058015.</ref> In this unusual species
- Xiao-Gang Wang; Tucker Carrington Jr (2016). "Calculated rotation-bending energy levels of CH5+ and a comparison with experiment". Journal of Chemical Physics. 144 (20): 204304. Bibcode:2016JChPh.144t4304W. doi:10.1063/1.4948549. PMID 27250303.
- H. Schmiedt; Per Jensen; S. Schlemmer (2017). "Rotation-vibration motion of extremely flexible molecules - The molecular superrotor". Chemical Physics Letters. 672: 34–46. Bibcode:2017CPL...672...34S. doi:10.1016/j.cplett.2017.01.045.
See also[edit]
References[edit]
- ^ Chemistry, International Union of Pure and Applied (2009). "carbonium ion". IUPAC Compendium of Chemical Terminology. IUPAC. doi:10.1351/goldbook.C00839. ISBN 978-0-9678550-9-7. Retrieved 27 November 2018.
- ^ V. L. Talrose and A. K. Lyubimova, Dokl. Akad. Nauk SSSR 86, 909-912 (1952) (In Russian: Тальрозе, В. Л., and А. К. Любимова. "Вторичные процессы в ионном источнике масс-спектрометра." ДАН СССР 86 (1952): 909-912)
- ^ Nikolaev, Eugene (1998). "Victor Talrose: an appreciation". Journal of Mass Spectrometry. 33 (6): 499–501. Bibcode:1998JMSp...33..499N. doi:10.1002/(SICI)1096-9888(199806)33:6<499::AID-JMS684>3.0.CO;2-C. ISSN 1076-5174.
- ^ Rasul, Golam; Prakash, G.K. Surya; Olah, George A. (2011). "Comparative study of the hypercoordinate carbonium ions and their boron analogs: A challenge for spectroscopists". Chemical Physics Letters. 517 (1–3): 1–8. Bibcode:2011CPL...517....1R. doi:10.1016/j.cplett.2011.10.020.
- ^ Schreiner, Peter R.; Kim, Seung-Joon; Schaefer, Henry F.; von Ragué Schleyer, Paul (1993). "CH+
5: The never-ending story or the final word?". Journal of Chemical Physics. 99 (5): 3716–3720. doi:10.1063/1.466147. - ^ Müller, Hendrik; Kutzelnigg, Werner; Noga, Jozef; Klopper, Wim (1997). "CH5+: The story goes on. An explicitly correlated coupled-cluster study". Journal of Chemical Physics. 106 (5): 1863. Bibcode:1997JChPh.106.1863M. doi:10.1063/1.473340.
- ^ White, Edmund T.; Tang, Jian; Oka, Takeshi (1999). "CH+
5: The infrared spectrum observed". Science. 284 (5411): 135–7. Bibcode:1999Sci...284..135W. doi:10.1126/science.284.5411.135. PMID 10102811. - ^ Oskar Asvany, Padma Kumar P; Redlich, Britta; Hegemann, Ilka; Schlemmer, Stephan; Marx, Dominik (2005). "Understanding the infrared spectrum of bare CH+
5". Science. 309 (5738): 1219–1222. Bibcode:2005Sci...309.1219A. doi:10.1126/science.1113729. PMID 15994376. S2CID 28745636. - ^ Huang, Xinchuan; McCoy, Anne B.; Bowman, Joel M.; Johnson, Lindsay M.; Savage, Chandra; Dong, Feng; Nesbitt, David J. (2006). "Quantum deconstruction of the infrared spectrum of CH+
5". Science. 311 (5757): 60–63. Bibcode:2006Sci...311...60H. doi:10.1126/science.1121166. PMID 16400143. S2CID 26158108. - ^ Sommer, J.; Jost, R. (2000). "Carbenium and carbonium ions in liquid- and solid-superacid-catalyzed activation of small alkanes" (PDF). Pure and Applied Chemistry. 72 (12): 2309–2318. doi:10.1351/pac200072122309. S2CID 46627813.
- ^ a b Field, F. H.; Munson, M. S. B. (1965). "Reactions of gaseous ions. XIV. Mass spectrometric studies of methane at pressures to 2 Torr". Journal of the American Chemical Society. 87 (15): 3289–3294. doi:10.1021/ja01093a001.