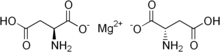 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.038.806 |
| Chemical and physical data | |
| Formula | C8H12MgN2O8 |
| Molar mass | 288.495 g·mol−1 |
| | |
Magnesium aspartate, the chelated magnesium salt of aspartic acid, it is a mineral supplement.[1]
Chemical action[edit]
Magnesium aspartate hydrochloride, sold under the Maginex DS brand, among others, contains approximately 10% elemental magnesium.[2] This means, for example, that there is 122 mg of magnesium in 1,230 mg Maginex DS dietary supplement granules.[2]
This form of magnesium supplementation has increased bioavailability compared to magnesium oxide and magnesium citrate. There were some promising clinical trials conducted in the 1960s that found a combination of magnesium and potassium aspartates had a positive effect on fatigue and they reduced muscle hyper-excitability.[3]
In its evaluation in 2005, the AFC Panel concluded that in humans the bioavailability of magnesium from magnesium L-aspartate was similar to that from other organic magnesium salts and the more soluble inorganic magnesium salts.[4] Overall, it was concluded that organic salts of magnesium have the greatest water solubility and demonstrate a greater oral absorption and bioavailability compared to less soluble magnesium preparations such as magnesium oxide, magnesium hydroxide, magnesium carbonate and magnesium sulphate.[5]
Chemical structure and Properties[edit]
Magnesium aspartate is a compound formed by the combination of the divalent magnesium cation (Mg2+) and the dicarboxylic amino acid aspartate (C4H6NO4-). The chemical formula for this compound is Mg(C4H6NO4)2. [6]
The structure of magnesium aspartate consists of a central magnesium ion that is chelated, or bound, by two aspartate anions. The aspartate moiety contains a carboxyl group (-COOH), an amino group (-NH2), and a second carboxyl group, forming a dicarboxylic amino acid structure. [6]
This unique chelated structure is responsible for the enhanced water solubility of magnesium aspartate compared to other magnesium salts, such as magnesium oxide or magnesium citrate. The water-soluble nature of the compound is a key factor contributing to its improved bioavailability when taken orally.[6]
The chelation of the magnesium ion by the two aspartate groups facilitates the efficient absorption and utilization of magnesium by the body. The aspartate moiety acts as a chelating agent, helping to transport the magnesium ions across cell membranes and into the bloodstream, where they can be distributed to various tissues and organs.
This improved absorption and distribution of magnesium is a significant advantage of using magnesium aspartate as a supplement. The chelated structure enhances the bioavailability of the magnesium, allowing for better utilization by the body compared to other less soluble and less bioavailable forms of magnesium.
The structure of magnesium aspartate, with the divalent magnesium cation chelated by two aspartate anions, contributes to its high water solubility and improved bioavailability. This unique structural feature is a key factor in the enhanced absorption and utilization of magnesium from this particular form of magnesium supplementation.
Clinical Applications and Benefits[edit]
Magnesium aspartate has been investigated for its potential therapeutic applications in various health conditions. Some of the areas where magnesium aspartate has shown promise include:
- Cardiovascular Health
Numerous studies have suggested that magnesium aspartate may have beneficial effects on cardiovascular health. It has been explored for its potential role in reducing blood pressure, improving endothelial function, and reducing the risk of arrhythmias and other heart-related conditions[7]
- Diabetes and Metabolic Disorders
Magnesium aspartate has been studied for its potential to improve insulin sensitivity and glycemic control in individuals with diabetes or metabolic disorders. The improved bioavailability of magnesium may contribute to these potential benefits.
a) Improved Insulin Sensitivity
Several studies have demonstrated that magnesium aspartate supplementation can enhance insulin sensitivity, even in individuals who are overweight, insulin resistant, and non-diabetic.[8][9]
The improved insulin sensitivity observed with magnesium aspartate supplementation may help compensate for variations in insulin sensitivity, potentially benefiting those with diabetes or metabolic disorders.[8]
b) Glycemic Control
Magnesium aspartate has been investigated for its ability to improve glycemic control in individuals with diabetes. Research has found that supplementation with 250-450 mg of magnesium aspartate daily for 6-24 weeks can significantly reduce fasting blood sugar levels compared to placebo groups.[6][9].This suggests that the improved bioavailability of magnesium from the aspartate compound may help optimize blood sugar regulation in those with diabetes.
- Improved Muscle Function
Magnesium is required for the proper contraction and relaxation of muscles. It acts as a cofactor for numerous enzymes involved in muscle function, energy production, and electrolyte balance.[8][9]
Magnesium aspartate supplementation has been shown to improve various measures of muscle performance, including grip strength, lower-leg power, knee extension torque, and ankle extension strength.[6][10]
- Enhanced Energy Metabolism
Magnesium is essential for the efficient conversion of food into usable energy through processes like glycolysis and the Krebs cycle.[8][9]Animal studies have indicated that magnesium aspartate can enhance glucose availability in the brain, muscles, and blood, potentially improving exercise efficiency and delaying the onset of fatigue.[6],[11].
- Improved Recover and Endurance
Magnesium aspartate may also play a role in supporting exercise recovery and endurance. By helping to regulate muscle function and energy metabolism, magnesium aspartate supplementation has been associated with improvements in measures like maximal isometric trunk flexion, rotation, and jumping performance[6][10]
Dosage[edit]
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| Birth to 6 months | 30 mg* | 30 mg* | ||
| 7–12 months | 75 mg* | 75 mg* | ||
| 1–3 years | 80 mg | 80 mg | ||
| 4–8 years | 130 mg | 130 mg | ||
| 9–13 years | 240 mg | 240 mg | ||
| 14–18 years | 410 mg | 360 mg | 400 mg | 360 mg |
| 19–30 years | 400 mg | 310 mg | 350 mg | 310 mg |
| 31–50 years | 420 mg | 320 mg | 360 mg | 320 mg |
| 51+ years | 420 mg | 320 mg |
- Adequate Intake (AI)
Magnesium-L-aspartate 1230 mg (magnesium 122 mg) up to 3 times/day Dosage adjustment in renal impairment: Patients with severe kidney failure should not receive magnesium due to toxicity from accumulation.[13]
Magnesium supplements and other magnesium containing products, such as antacids, can bind with prescription medicines, reducing their effectiveness.[14]
Safety[edit]
When considering aspartate sources individually, the levels of exposure estimated in this opinion amount up to 6 g/day for calcium aspartate (equivalent to 100 mg/kg bw/day for a 60 kg individual), 5.8 g/day for magnesium aspartate (equivalent to 97 mg/kg bw/day), 4 g/day for potassium aspartate (equivalent to 67 mg/kg bw/day), 0.05 g/day for zinc aspartate (0.8 mg/kg bw/day) and 0.008 g/day for copper aspartate (0.1 mg/kg bw/day). These values are all below those reported to induce amino acid imbalance in intervention trials (6.3 g aspartate/day) and they are, respectively, 7, 7.2, 10.5, 875 and 7000 times lower than the NOAEL for aspartate identified from a 90-day rat study. Based on these margins of safety, the Panel concludes that the use of zinc and copper aspartate, as sources of zinc and copper at the proposed use levels, are not of safety concern but that the use of calcium, magnesium and potassium aspartate could be of safety concern because the margins of safety are considered too low. The Panel notes that if all sources would be used simultaneously, combined exposure will be 16 g/day (equivalent to 267 mg/kg bw/day), which is above the reported amounts inducing amino acid imbalance in intervention trials (6.3 g/day). Furthermore, this value is only 3 times lower than the NOAEL from the rat study and due to the low margin of safety the Panel considers this of safety concern. The Panel estimates that the exposure to aspartate from these food supplements should be added to the aspartate exposure arising from the diet.
Based on US data, estimates of the mean exposure to aspartic acid arising from the diet are 4.1 g/day (children 1-3 year old) to 9.3 g/day (males 19-30 year old) and at the 95th percentile 6.6 g/day (children 4-8 year old) to 12.9 g/day (males 19-50 year old). Under these conditions, estimates of maximum daily exposure to aspartate ions from the diet (13 g/day) and from calcium or magnesium aspartate supplements would be approximately 19 g/day6, and from potassium aspartate would be 17 g/day.[15] Aspartate exposure estimates from zinc or copper supplementation would not significantly change aspartate exposure from the diet.
Taken individually these levels of exposure are lower than those reported to induce amino acid imbalance in intervention trials, when aspartate exposure from the diet is also taken into consideration (19.3 g/day).[16] However, when considering the potential total intake of aspartic ions arising from the diet and from a potential multi-mineral combination of all food supplements the exposure could add up to 29 g/day.[17] In line with the SCF concerns, the Panel considers that the use of L-amino acids in food supplements should not give rise to a nutritional imbalance of the amino acids. Thus the Panel concludes that under these conditions aspartate ion exposure from a multi-mineral combination of this type could be of safety concern.[5]
References[edit]
- ^ "Magnesium L-aspartate". PubChem. Retrieved 31 December 2015.
- ^ a b Guerrera MP, Volpe SL, Mao JJ (15 July 2009). "Therapeutic Uses of Magnesium". American Family Physician. 80 (2): 157–162. PMID 19621856.
- ^ Ianna M. "Understanding Different Types of Magnesium". drnibber.com. Retrieved 31 December 2015.
- ^ "Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Foods on a request from the Commission related to Magnesium Aspartate as a mineral substance used as a source of magnesium in dietary foods for special medical purposes". The EFSA Journal. 167: 1–6. 2005.
- ^ a b "Magnesium aspartate, potassium aspartate, magnesium potassium aspartate, calcium aspartate, zinc aspartate, and copper aspartate as sources for magnesium, potassium, calcium, zinc, and copper added for nutritional purposes to food supplements" (PDF). The EFSA Journal. 883: 1–23. 2008. Retrieved 31 December 2015 – via efsa.europa.eu.
- ^ a b c d e f g Ranade VV, Somberg JC (September 2001). "Bioavailability and Pharmacokinetics of Magnesium After Administration of Magnesium Salts to Humans". American Journal of Therapeutics. 8 (5): 345–357. doi:10.1097/00045391-200109000-00008. ISSN 1075-2765. PMID 11550076.
- ^ Schwalfenberg GK, Genuis SJ (2017). "The Importance of Magnesium in Clinical Healthcare". Scientifica. 2017: 1–14. doi:10.1155/2017/4179326. ISSN 2090-908X. PMC 5637834. PMID 29093983.
- ^ a b c d Gröber U, Schmidt J, Kisters K (2015-09-23). "Magnesium in Prevention and Therapy". Nutrients. 7 (9): 8199–8226. doi:10.3390/nu7095388. ISSN 2072-6643. PMC 4586582. PMID 26404370.
- ^ a b c d Castiglioni S, Cazzaniga A, Albisetti W, Maier J (2013-07-31). "Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions". Nutrients. 5 (8): 3022–3033. doi:10.3390/nu5083022. ISSN 2072-6643. PMC 3775240. PMID 23912329.
- ^ a b M�hlbauer B., Schwenk M, Coram WM, Antonin KH, Etienne P, Bieck PR, Douglas FL (April 1991). "Magnesium-L-aspartate-HCl and magnesium-oxide: bioavailability in healthy volunteers". European Journal of Clinical Pharmacology. 40 (4): 437–438. doi:10.1007/bf00265863. ISSN 0031-6970. PMID 2050185.
{{cite journal}}: Vancouver style error: non-Latin character in name 1 (help); replacement character in|last1=at position 2 (help) - ^ "The Science Behind the Music– Performance Connection". Applying Music in Exercise and Sport: 21–42. 2007. doi:10.5040/9781492595229.ch-002. ISBN 978-1-4925-9522-9.
- ^ "Magnesium Fact Sheet for Health Professionals". NIH. Retrieved 31 December 2015.
- ^ "Magnesium L-aspartate Hydrochloride". Baltimore Washington Health Center. Univ. of Maryland. Retrieved 31 December 2015.
- ^ "magnesium aspartate HCl". WebMD. Retrieved 31 December 2015.
- ^ "Technical dossier on DL-magnesium-aspartate-tetrahydrate. February 2005g. Submitted by Gradiens Ltd. Budapest, Hungary."
- ^ "Technical dossier on magnesium L-aspartate. May 2005h. Submitted by Health Food Manufacturer’s Association. Surrey, England."
- ^ "Technical dossier on magnesium L-aspartat e. June 2005i. Submitted by Kiwi Farm b.v. Katwijk, Nederland."