| Calcium channel, voltage-dependent | |
|---|---|
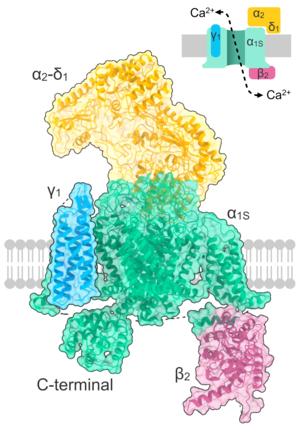 Crystallographic structure of the L-type calcium channel complex (subunits α1S, α2, δ, β, and γ). | |
| Identifiers | |
| Symbol | Calcium channel, voltage-dependent |


The L-type calcium channel (also known as the dihydropyridine channel, or DHP channel) is part of the high-voltage activated family of voltage-dependent calcium channel.[2] "L" stands for long-lasting referring to the length of activation. This channel has four isoforms: Cav1.1, Cav1.2, Cav1.3, and Cav1.4.
L-type calcium channels are responsible for the excitation-contraction coupling of skeletal, smooth, cardiac muscle, and for aldosterone secretion in endocrine cells of the adrenal cortex.[1] They are also found in neurons, and with the help of L-type calcium channels in endocrine cells, they regulate neurohormones and neurotransmitters. They have also been seen to play a role in gene expression, mRNA stability, neuronal survival, ischemic-induced axonal injury, synaptic efficacy, and both activation and deactivation of other ion channels.[3]
In cardiac myocytes, the L-type calcium channel passes inward Ca2+ current (ICaL) and triggers calcium release from the sarcoplasmic reticulum by activating ryanodine receptor 2 (RyR2) (calcium-induced-calcium-release).[4] Phosphorylation of these channels increases their permeability to calcium and increases the contractility of their respective cardiac myocytes.
L-type calcium channel blocker drugs are used as cardiac antiarrhythmics or antihypertensives, depending on whether the drugs have higher affinity for the heart (the phenylalkylamines, like verapamil), or for the blood vessels (the dihydropyridines, like nifedipine).[5]
In skeletal muscle, there is a very high concentration of L-type calcium channels, situated in the T-tubules. Muscle depolarization results in large gating currents, but anomalously low calcium flux, which is now explained by the very slow activation of the ionic currents. For this reason, little or no Ca2+ passes across the T-tubule membrane during a single action potential.
History[edit]
In 1953, Paul Fatt and Bernard Katz discovered voltage gated calcium channels in crustacean muscle. The channels exhibited different activation voltages and calcium conducting properties and were thus separated into High Voltage Activating channels (HVA) and Low Voltage Activating channels (LVA). After further experimentation, it was found that HVA channels were blocked by derivatives of 1,4-dihydropyridine (DHPs).[6] Using DHPs, it was found that HVA channels were specific to certain tissues and reacted differently, which led to further categorization of the HVA channels into L-type, P-type, and N-type.[3] L-type calcium channels were peptide sequenced and it was found that there were 4 kinds of L-type calcium channels: α1S (Skeletal Muscle), α1C (Cardiac), α1 D (found in the brain), and α1F (found in the retina).[6] In 2000, after more research was done on α1 subunits in voltage-gated calcium channels, a new nomenclature was used that called L-type calcium channels CaV1 with its subunits being called CaV1.1, Cav1.2, CaV1.3, and CaV1.4.[3] Research on the CaV1 subunits continues to reveal more about their structure, function, and pharmaceutical applications.[7]
Structure[edit]
L-type Calcium Channels contain 5 different subunits, the α1(170–240 kDa), α2(150kDa), δ(17-25 kDa), β(50-78 kDa), and γ(32 kDa) subunits.[8] The α2, δ, and β subunits are non-covalently bonded to the α1 subunit and modulate ion trafficking and biophysical properties of the α1 subunit. The α2 and δ subunits are in the extracellular space while the β and γ subunits are located in the cytosolic space.[8]
The α1 subunit is a heterotetramer that has four transmembrane regions, known as Domains I-IV, that cross the plasma six times as α-helices, being called S0-S6 (S0 and S1 together cross the membrane once).[3] The α1 subunit as a whole contains the voltage sensing domain, the conduction pore, and gating apparatus.[9] Like most voltage-gated ion channels, the α-subunit is composed of 4 subunits. Each subunit is formed by 6 alpha-helical, transmembrane domains that cross the membrane (numbered S1-S6). The S1-S4 subunits make up the voltage sensor, while S5-S6 subunits make up the selectivity filter.[10] To sense the cell's voltage, the S1-S3 helices contain many negatively charged amino acids while S4 helices contain mostly positively charged amino acids with a P-loop connecting the S4 to S5 helices. After the S1-6 domains, there are six C domains that consist of two EF-hand motifs (C1-2 and C3-4) and a Pre-IQ domain (C5) and IQ domain (C6). There are also two EF-hand motifs on the N-terminus. Both the N and C terminus are in the cytosolic space with the C-terminus being much longer than the N-terminus.[11]
The β subunit is known to have four isoforms (β1-β4) to regulate the channel's functions and is connected to α1 through the α1 I and II linker in the cytosol at the β α1-binding pocket (ABP).[7][12] Each isoform contains a src homology 3 domain (SH3) and a guanylate-kinase like domain (GK) that are separated by a HOOK domain, and three unstructured regions.[12]
The α2 and δ subunits are connected together by disulfide bonds (sometimes known as the α2δ subunit) and interact with α1.[7] they have four known isoforms called α2δ-1 to α2δ-2 and contain a von Willebrand A (VWA) domain and a Cache domain. The α2 region is in the extracellular space while the δ region is in the cell membrane and have been seen to be anchored with a glycosylphosphatidylinositol (GPI) anchor.[12]
The γ subunit has eight isoforms (γ1-γ8) and is connected to the α1 subunit and has only been found in muscle cells in the CaV1.1 and CaV1.2 channels.[12] Not much is known about the γ subunit, but it has been linked to interactions in hydrophobic forces.[3]
Mechanism[edit]
Opening of the pore in L-type calcium channels takes place in the α1 subunit. When the membrane depolarizes, the S4 helix moves through the S4 and S5 linkers to the cytoplasmic ends of the S5 and S6 helices. This opens the activation gate which is formed by the inner side of the S6 helices in the α1 subunit.[11]
The most predominant way of autoinhibition of L-type calcium channels is with the Ca2+/Cam complex.[11] As the pore opens and causes an influx of calcium, calcium binds to calmodulin and then interacts with the loop that connects the adjacent EF-hand motifs and causes a conformational change in the EF-hand motif so it interacts with the pore to cause quick inhibition in the channel.[6] It is still debated on where and how the pore and EF-hand interact. Hydrophobic pockets in the Ca2+/Cam complex will also bind to three sections of the IQ domain known as the “aromatic anchors”.[11] The Ca2+/Cam complex has a high affinity towards L-type calcium channels, allowing it to get blocked even when there are low amounts of calcium present in the cell. The pore eventually closes as the cell repolarizes and causes a conformational change in the channel to put it in the closed conformation.
Inhibition and modulation[edit]
One of the most recognized characteristics of the L-type calcium channel is its unique sensitivity to 1,4-dihydropyridines (DHPs).[3] Unlike other voltage gated calcium channels, L-type calcium channels are resistant to ⍵-CT X (GVIA) and ⍵-AG A (IVA) inhibitory drugs.[3]
A well observed form of modulation is due to alternative splicing. A common form of modulation from alternative splicing is the C-terminal modulator (CTM). It has a positively charged α-helix on the C-terminal called the DCRD and a negatively charged helix right after the IQ motif (CaM interaction site) called the PCRD. The two helices can form a structure that bind competitively with CaM to reduce the open-state probability and lower calcium-dependent inhibition (CDI).[7]
Alternative splicing is also seen on the β subunits to create different isoforms to give channels different properties due to palmitoylation[6] and RNA editing.[7] Other forms of modulation on the β subunit include increasing or decreasing of the subunit's expression. This is due to the fact that β subunits increase the open-probability of the channel, activity in the plasma membrane, and antagonize the ubiquitination of the channel.[6]
L-type calcium channels are also modulated by G protein-coupled receptors and the adrenergic nervous system.[6] Protein Kinase A (PKA) activated by a G protein-coupled receptors cascade can phosphorylate L-type calcium channels, after channels form a signaling complex with A-Kinase-Anchoring proteins (AKAPs) , to increase calcium current through the channel, increasing the open-state probability, and an accelerated recovery period. Activated Phospholipase C (PLC) from G protein-coupled receptors can breakdown polyphosphoinositides to decrease the channel's calcium current by 20%-30%.[7]
The adrenergic nervous system has been seen to modulate L-type calcium channels by cleaving the C-terminal fragment when the β-adrenergic receptor is stimulated to increase activation of the channels.[6]
Genes[edit]
See also[edit]
References[edit]
- ^ a b Felizola SJ, Maekawa T, Nakamura Y, Satoh F, Ono Y, Kikuchi K, et al. (October 2014). "Voltage-gated calcium channels in the human adrenal and primary aldosteronism". The Journal of Steroid Biochemistry and Molecular Biology. 144 Pt B (part B): 410–416. doi:10.1016/j.jsbmb.2014.08.012. PMID 25151951. S2CID 23622821.
- ^ Rossier MF (2016). "T-Type Calcium Channel: A Privileged Gate for Calcium Entry and Control of Adrenal Steroidogenesis". Frontiers in Endocrinology. 7: 43. doi:10.3389/fendo.2016.00043. PMC 4873500. PMID 27242667.
- ^ a b c d e f g Lipscombe D, Helton TD, Xu W (November 2004). "L-type calcium channels: the low down". Journal of Neurophysiology. 92 (5): 2633–2641. doi:10.1152/jn.00486.2004. PMID 15486420.
- ^ Yamakage M, Namiki A (February 2002). "Calcium channels--basic aspects of their structure, function and gene encoding; anesthetic action on the channels--a review". Canadian Journal of Anaesthesia. 49 (2): 151–164. doi:10.1007/BF03020488. PMID 11823393.
- ^ Hughes A (2017). "Calcium channel blockers". In Bakris G, Sorrentino M (eds.). Hypertension: a companion to Braunwald's heart disease (Third ed.). Philadelphia, PA: Elsevier Health Sciences. pp. 242–253. ISBN 9780323508766. OCLC 967938982.
- ^ a b c d e f g Dolphin AC (October 2018). "Voltage-gated calcium channels: their discovery, function and importance as drug targets". Brain and Neuroscience Advances. 2: 2398212818794805. doi:10.1177/2398212818794805. PMC 6179141. PMID 30320224.
- ^ a b c d e f Striessnig J, Pinggera A, Kaur G, Bock G, Tuluc P (March 2014). "L-type Ca2+ channels in heart and brain". Wiley Interdisciplinary Reviews. Membrane Transport and Signaling. 3 (2): 15–38. doi:10.1002/wmts.102. PMC 3968275. PMID 24683526.
- ^ a b Bodi I, Mikala G, Koch SE, Akhter SA, Schwartz A (December 2005). "The L-type calcium channel in the heart: the beat goes on". The Journal of Clinical Investigation. 115 (12): 3306–3317. doi:10.1172/JCI27167. PMC 1297268. PMID 16322774.
- ^ "Voltage-gated calcium channels | Introduction | BPS/IUPHAR Guide to PHARMACOLOGY". www.guidetopharmacology.org. Retrieved 2019-11-28.
- ^ Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J (December 2005). "International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels". Pharmacological Reviews. 57 (4): 411–425. doi:10.1124/pr.57.4.5. PMID 16382099. S2CID 10386627.
- ^ a b c d Wahl-Schott C, Baumann L, Cuny H, Eckert C, Griessmeier K, Biel M (October 2006). "Switching off calcium-dependent inactivation in L-type calcium channels by an autoinhibitory domain". Proceedings of the National Academy of Sciences of the United States of America. 103 (42): 15657–15662. Bibcode:2006PNAS..10315657W. doi:10.1073/pnas.0604621103. PMC 1622877. PMID 17028172.
- ^ a b c d Shaw RM, Colecraft HM (May 2013). "L-type calcium channel targeting and local signalling in cardiac myocytes". Cardiovascular Research. 98 (2): 177–186. doi:10.1093/cvr/cvt021. PMC 3633156. PMID 23417040.
Further reading[edit]
- Takahashi K, Hayashi S, Miyajima M, Omori M, Wang J, Kaihara K, et al. (May 2019). "L-type calcium channel modulates mechanosensitivity of the cardiomyocyte cell line H9c2". Cell Calcium. 79: 68–74. doi:10.1016/j.ceca.2019.02.008. PMID 30836292.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
External links[edit]
- "Voltage-Gated Calcium Channels". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.
- L-Type+Calcium+Channel at the U.S. National Library of Medicine Medical Subject Headings (MeSH)