 | |
| Clinical data | |
|---|---|
| Other names | β-hydroxythiofentanyl |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
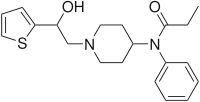
| Formula | C20H26N2O2S |
| Molar mass | 358.50 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Betahydroxythiofentanyl (β-hydroxythiofentanyl) is an opioid analgesic that is an analog of fentanyl.
Betahydroxythiofentanyl was sold briefly on the black market from around 1985,[1] before the introduction of the Federal Analog Act in 1986 which for the first time attempted to control entire families of drugs based on their structural similarity rather than scheduling each drug individually as they appeared.[2] β-hydroxythiofentanyl was anecdotally said to be one of the more favored fentanyl analogs by opiate addicts[citation needed].
Side Effects[edit]
Betahydroxythiofentanyl has effects and side effects similar to fentanyl. Side effects of fentanyl analogs include itching, nausea and potentially serious respiratory depression, which can be life-threatening.[citation needed]
Legal status[edit]
As of October 2015, betahydroxythiofentanyl is a controlled substance in China.[3]
As of May 2016, betahydroxythiofentanyl was temporarily listed as a Schedule I controlled substance in the United States.[4] A final ruling placing it in Schedule I was issued by the DEA on May 8, 2019 after a 1 year notice of proposed permanent scheduling.[5] There were no petitions for hearings on the matter.[6][7]
See also[edit]
References[edit]
- ^ Valter K, Arrizabalaga P. Designer Drugs Directory (1998), p150. ISBN 0-444-20525-X
- ^ Henderson GL (1988). "Designer Drugs: Past History and Future Prospects". Journal of Forensic Sciences. 33 (2): 569–575. doi:10.1520/JFS11976J. PMID 3286815.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- ^ "Schedules of Controlled Substances: Temporary Placement of Butyryl Fentanyl and Beta-Hydroxythiofentanyl into Schedule I" (PDF). Drug Enforcement Administration. 12 May 2016.
- ^ 5 U.S.C. § 556-557
- ^ Drug Enforcement Administration (May 8, 2019). "Schedules of Controlled Substances: Placement of beta-Hydroxythiofentanyl in Schedule I". Federal Register. 84 (89). Department of Justice: 20023–20027. Docket No. DEA–484. Retrieved 7 March 2024.
- ^ 21 CFR 1308.11