| |

| |
| Names | |
|---|---|
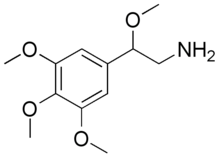
| Preferred IUPAC name
2-Methoxy-2-(3,4,5-trimethoxyphenyl)ethan-1-amine | |
| Other names
3,4,5,beta-Tetramethoxyphenethylamine
2-(3,4,5,beta-Tetramethoxyphenyl)ethanamine β-methoxymescaline | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H19NO4 | |
| Molar mass | 241.287 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
BOM (3,4,5,beta-tetramethoxyphenethylamine) is a lesser-known psychedelic drug. It is the beta-methoxy derivative of mescaline. BOM was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 200 mg, and the duration unknown.[1] BOM produces few to no effects.[2] Very little data exists about its pharmacological properties, metabolism, and toxicity.
Legality[edit]
United Kingdom[edit]
This substance is a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act.[3]
See also[edit]
References[edit]
- ^ BOM Entry in PiHKAL
- ^ Shulgin, Alexander; Shulgin, Ann (September 1991). PiHKAL: A Chemical Love Story. Berkeley, California: Transform Press. ISBN 0-9630096-0-5. OCLC 25627628.
- ^ "UK Misuse of Drugs act 2001 Amendment summary". Isomer Design. Retrieved 12 March 2014.