| |
| Clinical data | |
|---|---|
| Routes of administration | By mouth, insufflation, vaporization, IV |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
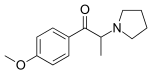
| Formula | C14H19NO2 |
| Molar mass | 233.311 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
4'-Methoxy-α-pyrrolidinopropiophenone (MOPPP) is a stimulant designer drug of the pyrrolidinophenone class. It has the potential to produce euphoria,[citation needed] an effect shared with other classical stimulants.
Recreational use[edit]
MOPPP use is infrequent when compared to other amphetamines or stimulants used recreationally, such as meth, cocaine, or speed. It first arose as a designer drug in Germany in the late 1990s and early 2000s,[1][2][3] along with a number of other derivatives but never gained the international popularity[4][5] that other drugs in its family of pyrrolidinophenone derivatives had (such as α-PPP and MDPV).
While the recent trend of selling stimulants through false labeling (i.e., bath salts) has gained notoriety, using MDPV as its main ingredient, this has not been the case with MOPPP, despite its similar potential for abuse.[6]
Chemistry[edit]
MOPPP is structurally related to α-PPP in the same way that PMA is related to amphetamine: a methoxy group has been added to the 4-position on the phenyl ring.
Metabolism[edit]
MOPPP appears to be metabolized within the liver chiefly by the enzyme CYP2D6.[2]
See also[edit]
- α-Pyrrolidinopropiophenone (α-PPP)
- 4'-Methyl-α-pyrrolidinopropiophenone (MPPP)
- 3,4-Methylenedioxy-α-pyrrolidinopropiophenone (MDPPP)
- 3',4'-Methylenedioxy-α-pyrrolidinobutiophenone (MDPBP)
- Methylenedioxymethamphetamine (MDMA) or Ecstasy, Molly
- Trifluoromethylphenylpiperazine (TMPPP)
References[edit]
- ^ Springer D, Fritschi G, Maurer HH (August 2003). "Metabolism and toxicological detection of the new designer drug 4'-methoxy-alpha-pyrrolidinopropiophenone studied in rat urine using gas chromatography-mass spectrometry". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 793 (2): 331–42. doi:10.1016/S1570-0232(03)00334-9. PMID 12906908.
- ^ a b Springer D, Staack RF, Paul LD, Kraemer T, Maurer HH (October 2003). "Identification of cytochrome P450 enzymes involved in the metabolism of 4'-methoxy-alpha-pyrrolidinopropiophenone (MOPPP), a designer drug, in human liver microsomes". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 33 (10): 989–98. doi:10.1080/00498250310001602775. PMID 14555336. S2CID 7838464.
- ^ Springer D, Fritschi G, Maurer HH (November 2003). "Metabolism of the new designer drug alpha-pyrrolidinopropiophenone (PPP) and the toxicological detection of PPP and 4'-methyl-alpha-pyrrolidinopropiophenone (MPPP) studied in rat urine using gas chromatography-mass spectrometry". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences. 796 (2): 253–66. doi:10.1016/j.jchromb.2003.07.008. PMID 14581066.
- ^ Maurer HH, Kraemer T, Springer D, Staack RF (April 2004). "Chemistry, pharmacology, toxicology, and hepatic metabolism of designer drugs of the amphetamine (ecstasy), piperazine, and pyrrolidinophenone types: a synopsis". Therapeutic Drug Monitoring. 26 (2): 127–31. doi:10.1097/00007691-200404000-00007. PMID 15228152. S2CID 9255084.
- ^ Staack RF, Maurer HH (June 2005). "Metabolism of designer drugs of abuse". Current Drug Metabolism. 6 (3): 259–74. doi:10.2174/1389200054021825. PMID 15975043.
- ^ "MDPV Bath Salts Drug Over The Counter". Healthbodydaily.com. 8 March 2011. Archived from the original on 10 March 2011.