| |
| |
| Names | |
|---|---|
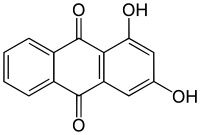
| Preferred IUPAC name
1,3-Dihydroxyanthracene-9,10-dione | |
| Other names
Purpuroxanthin; Xanthopurpurin
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H8O4 | |
| Molar mass | 240.21 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,3-Dihydroxyanthraquinone, also called purpuroxanthin or xanthopurpurin, is an organic compound with formula C
14H
8O
4 that occurs in the plant Rubia cordifolia (Indian madder).[1] It is one of ten dihydroxyanthraquinone isomers. Its molecular structure can be viewed as being derived from anthraquinone by replacement of two hydrogen atoms (H) by hydroxyl groups (-OH).
Xanthopurpurin occurs in small amounts (as a glycoside) in the root of the common madder plant, Rubia tinctorum, together with alizarin, purpurin and other anthraquinone derivatives.[2]
Properties[edit]
Xanthopurpurin is insoluble in hexane but soluble in chloroform. It can be obtained from solutions in the latter as reddish crystals that melt at 270–273 °C.[1]
Like many dihydroxy- and trihydroxyanthraquinones, it has a purgative action, although only 1/6 as effective as 1,2,7-trihidroxyanthraquinone (anthrapurpurin).[3]
See also[edit]
- alizarin (1,2-dihydroxyanthraquinone)
References[edit]
- ^ a b Padma S. Vankar, Rakhi Shanker, Debajit Mahanta and S.C. Tiwari (2008), Ecofriendly sonicator dyeing of cotton with Rubia cordifolia Linn. using biomordant. Dyes and Pigments, Volume 76, Issue 1, Pages 207-212. doi:10.1016/j.dyepig.2006.08.023
- ^ Goverdina C. H. Derksen, Harm A. G. Niederländer and Teris A. van Beek (2002), Analysis of anthraquinones in Rubia tinctorum L. by liquid chromatography coupled with diode-array UV and mass spectrometric detection. Journal of Chromatography A, Volume 978, Issues 1-2, Pages 119-127, doi:10.1016/S0021-9673(02)01412-7
- ^ Hugh Alister McGuigan (1921), An introduction to chemical pharmacology; pharmacodynamics in relation to chemistry. P. Blakiston's son, Philadelphia. Online version at archive.org, accessed on 2010-01-30.