| TUDOR domain | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
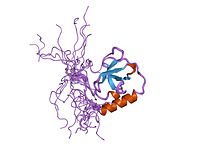 Structure of a TUDOR domain. | |||||||||||
| Identifiers | |||||||||||
| Symbol | TUDOR | ||||||||||
| Pfam | PF00567 | ||||||||||
| Pfam clan | CL0049 | ||||||||||
| InterPro | IPR008191 | ||||||||||
| SMART | TUDOR | ||||||||||
| PROSITE | PDOC50304 | ||||||||||
| SCOP2 | 3fdr / SCOPe / SUPFAM | ||||||||||
| CDD | cd04508 | ||||||||||
| |||||||||||
In molecular biology, a Tudor domain is a conserved protein structural domain originally identified in the Tudor protein encoded in Drosophila.[1] The Tudor gene was found in a Drosophila screen for maternal factors that regulate embryonic development or fertility.[2] Mutations here are lethal for offspring, inspiring the name Tudor, as a reference to the Tudor King Henry VIII and the several miscarriages experienced by his wives.[1]
Structure[edit]
A Tudor domain is a protein region approximately 60 amino acids in length, which folds into an SH3-like structure with a five-stranded antiparallel beta-barrel form.[1] Tudor domains can further be organized into functional units consisting of either a single Tudor domain, tandem Tudor domains, or hybrid Tudor domains consisting of two Tudor domains linked by an anti-parallel beta-sheet made from their shared second and third beta-strands.[1] An essential component of the Tudor domain structure is the aromatic-binding cage formed by several (typically 4–5) aromatic amino acid residues.[1]
Interaction with methylated residues[edit]
Tudor domains exert their functions by recognizing and binding methylated lysine and arginine residues, allowing them to function as histone readers in an epigenetic context.[1] This occurs through cation–pi interactions between the methylated Arg/Lys residue and the aromatic residues of the Tudor domain's aromatic-binding cage.[1] Depending on the Tudor domain, this interaction can be methylation state-specific (mono-, di-, or trimethylation).[1]
Function[edit]
DNA transcription and modification[edit]
Tudor domain proteins are involved in epigenetic regulation and can alter transcription by recognizing post-translational histone modifications and as adaptor proteins.[2] Recognition of methylated arginine and lysine histone residues results in the recruitment of downstream effectors, leading to chromatin silencing or activation depending on the Tudor domain protein and context.[1] For example, the human TDRD3 protein binds methylated arginine residues and promotes transcription of estrogen-responsive elements.[3] Conversely, the Polycomb-like protein (PCL) acts as an adaptor to recruit components of the Polycomb repressive complex 2 (PRC2), a histone H3K27 methyltransferase that represses transcription.[4] Additionally, Tudor domain proteins can repress transcription by recruiting DNA-methyltransferases to promote DNA methylation and heterochromatin assembly.[1] Tudor domain proteins also have the function of maintaining and propagating epigenetic modifications.[1]
Genome stability[edit]
The Tudor domain is involved in the silencing of selfish genetic elements, such as retrotransposons.[5] This functionality is performed both directly through Tudor-containing proteins, such as Tdrd7, as well as through piRNA synthesis.[6] Tudor domains are essential in the localization of protein machinery involved in piRNA creation, such as localization of Yb protein to the Yb body, assembly of the pole plasm in Drosophila, and recruitment of proteins to load Piwi with piRNA.[5]
DNA damage response[edit]
The human p53-binding protein 1 (TRP53BP1) is a Tudor domain protein involved in the DNA damage response (DDR) pathway, which functions to protect the genome from external stimuli.[5] It is a cascade of events that senses damage through adaptor proteins and triggers responses including cell cycle arrest, DNA repair, transcriptional modifications, and apoptosis.[5] TRP53BP1s Tudor domain mediates binding to sensors that accumulate at the sites of damage, and also functions as the adaptor promoting effector recruitment to the damaged sites.[5] TRP53BP1 is essential for DDR as it plays a very complex role in the regulation and recruitment of multiple other proteins involved.[5]
RNA metabolism[edit]
Tudor domain proteins involved in RNA metabolism have an extended Tudor domain of approximately 180 amino acids.[5] These proteins contain RNA-binding motifs to target RNAs, or bind to dimethylated arginines of proteins bound to RNAs.[5] These proteins regulate multiple aspects of RNA metabolism, including processing, stability, translation, and small RNA pathways.[5] Specifically, the survival motor neuron (SMN) protein is a Tudor domain protein that mediates the assembly of snRNPs (small nuclear ribonucleoproteins), by binding snRNAs and recruiting asymmetrically dimethylated arginines of SM proteins that form the protein constituent of snRNPs.[5] SMN promotes the maturation of snRNPs, which are essential for spliceosome assembly and intron removal.[5]
Examples[edit]

The proteins TP53BP1 (Tumor suppressor p53-binding protein 1) and its fission yeast homolog Crb2[8] and JMJD2A (Jumonji domain containing 2A) contain either tandem or double Tudor domains and recognize methylated histones.[9][10]
The structurally characterized Tudor domain in human SMN (survival of motor neuron) is a strongly bent anti-parallel β-sheet consisting of five β-strands with a barrel-like fold and recognizes symmetrically dimethylated arginine.[11]
Other Tudor domain containing proteins include AKAP1 (A-kinase anchor protein 1)[12] and ARID4A (AT rich interactive domain 4A) among others. A well known Tudor domain containing protein is Staphylococcal Nuclease Domain Containing 1 (SND1)/Tudor-SN/p100 co activator.[13] SND1 is involved in RISC complex and interacts with AEG-1 oncogene.[14] SND1 is also acts as an oncogene and plays a very important role in HCC and colon cancer.[15] The SND1 Tudor domain binds to methylated arginine in the PIWIL1 protein.[16] Tudor containing SND1 promotes tumor angiogenesis in human hepatocellular carcinoma through a novel pathway which involves NF-kappaB and miR-221.[17] Tudor SND1 is also present in the Drosophila melanogaster.[6]
References[edit]
- ^ a b c d e f g h i j k Botuyan MV, Mer G (2016). "Tudor Domains as Methyl-Lysine and Methyl-Arginine Readers". Chromatin Signaling and Diseases. Elsevier. pp. 149–165. doi:10.1016/b978-0-12-802389-1.00008-3. ISBN 978-0-12-802389-1.
- ^ a b Lu R, Wang GG (November 2013). "Tudor: a versatile family of histone methylation 'readers'". Trends in Biochemical Sciences. 38 (11): 546–55. doi:10.1016/j.tibs.2013.08.002. PMC 3830939. PMID 24035451.
- ^ Yang Y, Lu Y, Espejo A, Wu J, Xu W, Liang S, Bedford MT (December 2010). "TDRD3 is an effector molecule for arginine-methylated histone marks". Molecular Cell. 40 (6): 1016–23. doi:10.1016/j.molcel.2010.11.024. PMC 3090733. PMID 21172665.
- ^ Cai L, Rothbart SB, Lu R, Xu B, Chen WY, Tripathy A, et al. (February 2013). "An H3K36 methylation-engaging Tudor motif of polycomb-like proteins mediates PRC2 complex targeting". Molecular Cell. 49 (3): 571–82. doi:10.1016/j.molcel.2012.11.026. PMC 3570589. PMID 23273982.
- ^ a b c d e f g h i j k Pek JW, Anand A, Kai T (July 2012). "Tudor domain proteins in development". Development. 139 (13): 2255–66. doi:10.1242/dev.073304. PMID 22669818. S2CID 26275277.
- ^ a b Ying M, Chen D (January 2012). "Tudor domain-containing proteins of Drosophila melanogaster". Development, Growth & Differentiation. 54 (1): 32–43. doi:10.1111/j.1440-169x.2011.01308.x. PMID 23741747. S2CID 23227910.
- ^ Ozboyaci M, Gursoy A, Erman B, Keskin O (March 2011). "Molecular recognition of H3/H4 histone tails by the tudor domains of JMJD2A: a comparative molecular dynamics simulations study". PLOS ONE. 6 (3): e14765. Bibcode:2011PLoSO...614765O. doi:10.1371/journal.pone.0014765. PMC 3064570. PMID 21464980.
- ^ Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G (December 2006). "Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair". Cell. 127 (7): 1361–73. doi:10.1016/j.cell.2006.10.043. PMC 1804291. PMID 17190600.
- ^ Huang Y, Fang J, Bedford MT, Zhang Y, Xu RM (May 2006). "Recognition of histone H3 lysine-4 methylation by the double tudor domain of JMJD2A". Science. 312 (5774): 748–51. Bibcode:2006Sci...312..748H. doi:10.1126/science.1125162. PMID 16601153. S2CID 20036710.
- ^ Lee J, Thompson JR, Botuyan MV, Mer G (January 2008). "Distinct binding modes specify the recognition of methylated histones H3K4 and H4K20 by JMJD2A-tudor". Nature Structural & Molecular Biology. 15 (1): 109–11. doi:10.1038/nsmb1326. PMC 2211384. PMID 18084306.
- ^ Sprangers R, Groves MR, Sinning I, Sattler M (March 2003). "High-resolution X-ray and NMR structures of the SMN Tudor domain: conformational variation in the binding site for symmetrically dimethylated arginine residues". Journal of Molecular Biology. 327 (2): 507–20. doi:10.1016/s0022-2836(03)00148-7. PMID 12628254.
- ^ Rogne M, Landsverk HB, Van Eynde A, Beullens M, Bollen M, Collas P, Küntziger T (December 2006). "The KH-Tudor domain of a-kinase anchoring protein 149 mediates RNA-dependent self-association". Biochemistry. 45 (50): 14980–9. doi:10.1021/bi061418y. PMID 17154535.
- ^ Caudy AA, Ketting RF, Hammond SM, Denli AM, Bathoorn AM, Tops BB, et al. (September 2003). "A micrococcal nuclease homologue in RNAi effector complexes". Nature. 425 (6956): 411–4. Bibcode:2003Natur.425..411C. doi:10.1038/nature01956. PMID 14508492. S2CID 4410688.
- ^ Yoo BK, Santhekadur PK, Gredler R, Chen D, Emdad L, Bhutia S, et al. (May 2011). "Increased RNA-induced silencing complex (RISC) activity contributes to hepatocellular carcinoma". Hepatology. 53 (5): 1538–48. doi:10.1002/hep.24216. PMC 3081619. PMID 21520169.
- ^ Yoo BK, Emdad L, Lee SG, Su ZZ, Santhekadur P, Chen D, et al. (April 2011). "Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology". Pharmacology & Therapeutics. 130 (1): 1–8. doi:10.1016/j.pharmthera.2011.01.008. PMC 3043119. PMID 21256156.
- ^ Liu K, Chen C, Guo Y, Lam R, Bian C, Xu C, et al. (October 2010). "Structural basis for recognition of arginine methylated Piwi proteins by the extended Tudor domain". Proceedings of the National Academy of Sciences of the United States of America. 107 (43): 18398–403. Bibcode:2010PNAS..10718398L. doi:10.1073/pnas.1013106107. PMC 2972943. PMID 20937909.
- ^ Santhekadur PK, Das SK, Gredler R, Chen D, Srivastava J, Robertson C, et al. (April 2012). "Multifunction protein staphylococcal nuclease domain containing 1 (SND1) promotes tumor angiogenesis in human hepatocellular carcinoma through novel pathway that involves nuclear factor κB and miR-221". The Journal of Biological Chemistry. 287 (17): 13952–8. doi:10.1074/jbc.M111.321646. PMC 3340184. PMID 22396537.