 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Excretion | Renal (76%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.975 |
| Chemical and physical data | |
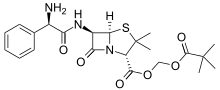
| Formula | C22H29N3O6S |
| Molar mass | 463.55 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Pivampicillin is a pivaloyloxymethyl ester of ampicillin. It is a prodrug, which is thought to enhance the oral bioavailability of ampicillin because of its greater lipophilicity compared to that of ampicillin.
Adverse effects[edit]
Prodrugs that release pivalic acid when broken down by the body—such as pivampicillin, pivmecillinam, and cefditoren pivoxil—have long been known to deplete levels of carnitine.[1][2] This effect is not due to the drug itself but to pivalate, which is mostly removed from the body by forming a conjugate with carnitine. Although short-term use of these drugs can cause a marked decrease in blood levels of carnitine,[3] it is unlikely to be of clinical significance;[2] long-term use, however, is not recommended.[2][4][5]
Availability[edit]
Worldwide, pivampicillin is only available in Denmark, where it is sold as Pondocillin® by PharmaCoDane, or Miraxid® by LEO Pharma.[6]
References[edit]
- ^ Holme E, Greter J, Jacobson CE, Lindstedt S, Nordin I, Kristiansson B, Jodal U (August 1989). "Carnitine deficiency induced by pivampicillin and pivmecillinam therapy". Lancet. 2 (8661): 469–473. doi:10.1016/S0140-6736(89)92086-2. PMID 2570185. S2CID 31555161.
- ^ a b c Brass EP (December 2002). "Pivalate-generating prodrugs and carnitine homeostasis in man". Pharmacological Reviews. 54 (4): 589–598. doi:10.1124/pr.54.4.589. PMID 12429869. S2CID 14507215.
- ^ Abrahamsson K, Holme E, Jodal U, Lindstedt S, Nordin I (June 1995). "Effect of short-term treatment with pivalic acid containing antibiotics on serum carnitine concentration--a risk irrespective of age". Biochemical and Molecular Medicine. 55 (1): 77–79. doi:10.1006/bmme.1995.1036. PMID 7551831.
- ^ Holme E, Jodal U, Linstedt S, Nordin I (September 1992). "Effects of pivalic acid-containing prodrugs on carnitine homeostasis and on response to fasting in children". Scandinavian Journal of Clinical and Laboratory Investigation. 52 (5): 361–372. doi:10.3109/00365519209088371. PMID 1514015.
- ^ Makino Y, Sugiura T, Ito T, Sugiyama N, Koyama N (September 2007). "Carnitine-associated encephalopathy caused by long-term treatment with an antibiotic containing pivalic acid". Pediatrics. 120 (3): e739–e741. doi:10.1542/peds.2007-0339. PMID 17724113. S2CID 40136171.
- ^ "Pondocillin®". Retrieved 2016-09-18.