| |
| Names | |
|---|---|
| IUPAC name
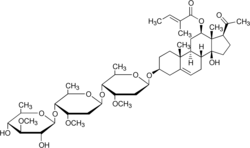
14-Hydroxy-3β-[(3-O-methyl-β-D-quinovopyranosyl)-(1→4)-(3-O-methyl-2-deoxy-β-D-ribo-hexopyranosyl)-(1→4)-3-O-methyl-2-deoxy-β-D-ribo-hexopyranosyloxy]-20-oxo-14β-pregn-5-en-12β-yl (2E)-2-methylbut-2-enoate
| |
| Systematic IUPAC name
(11S,13aS,13bR,17S,19aR,19bS,111R,111aS,32R,34S,35R,36R,52S,54S,55R,56R,72S,73R,74S,75R,76R)-11-Acetyl-13a,73,75-trihydroxy-34,54,74-trimethoxy-19a,111a,36,56,76-pentamethyl-12,13,13a,14b,14,16,17,18,19,19a,19b,110,111,111a-tetradecahydro-11H-2,4,6-trioxa-3,5(2,5),7(2)-tris(oxana)-1(7)-cyclopenta[a]phenanthrenaheptaphan-111-yl (2E)-2-methylbut-2-enoate | |
| Other names
P57
P57AS3 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C47H74O15 | |
| Molar mass | 879.094 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
P57 is an oxypregnane steroidal glycoside isolated from the African cactiform Hoodia gordonii. P57 is hypothesized to be the chemical constituent from this plant mainly responsible for the putative appetite suppressant activity of Hoodia extracts.[1][2][3]
In a study on rats at Brown Medical School, intracerebroventricular injections of the purified P57 demonstrated that the compound has a likely central nervous system (CNS) mechanism of action like that of neuroactive steroids. [4] The studies demonstrated that the compound increases the content of ATP by 50-150% in hypothalamic neurons. In addition, third ventricle administration of P57 reduced subsequent 24-hour food intake by 40-60%.
See also[edit]
- Anorectic, for additional information about appetite suppressants
- Neuroactive steroid
References[edit]
- ^ van Heerden FR, Marthinus Horak R, Maharaj VJ, Vleggaar R, Senabe JV, Gunning PJ (Oct 2007). "An appetite suppressant from Hoodia species". Phytochemistry. 68 (20): 2545–53. Bibcode:2007PChem..68.2545V. doi:10.1016/j.phytochem.2007.05.022. PMID 17603088.
- ^ van Heerden FR (Oct 2008). "Hoodia gordonii: a natural appetite suppressant". Journal of Ethnopharmacology. 119 (3): 134–137. doi:10.1016/j.jep.2008.08.023. PMID 18804523.
- ^ Avula, Bharathi; Yan-Hong Wang; Rahul S. Pawar; Yatin J. Shukla; Brian Schaneberg; Ikhlas A. Khan (May–June 2006). "Determination of the appetite suppressant P57 in Hoodia gordonii plant extracts and dietary supplements by liquid chromatography/electrospray ionization mass spectrometry (LC-MSD-TOF) and LC-UV methods". Journal of AOAC International. 89 (3): 606–611. doi:10.1093/jaoac/89.3.606. PMID 16792058. Retrieved 2006-07-11.
- ^ MacLean, David B.; Lu-Guang Luo (September 2004). "Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: studies of the anorectic mechanism of a plant steroidal glycoside". Brain Research. 1020 (1–2): 1–11. doi:10.1016/j.brainres.2004.04.041. PMID 15312781. S2CID 1994748.