 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.014.571 |
| Chemical and physical data | |
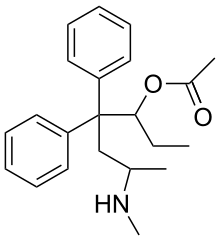
| Formula | C22H29NO2 |
| Molar mass | 339.479 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Noracymethadol (INN) is a synthetic opioid analgesic related to methadone that was never marketed.[2] In a clinical trial of postpartum patients it was reported to produce analgesia comparable to that of morphine but with less nausea, dizziness, and drowsiness.[3][4] Other side effects included salivation, ataxia, and respiratory depression that was reversible by naloxone.[3][4] Similarly to many of its analogues, noracymethadol is a Schedule I controlled substance in the United States with an ACSCN of 9633 and 2013 annual manufacturing quota of 12 grammes. [5] and is also controlled internationally under the United Nations Single Convention on Narcotic Drugs of 1961.[6] The salts known are the gluconate (free base conversion ratio 0.633) and hydrochloride (0.903).
Noracymethadol is an acetyl ester of methadol and it can be said with some precision that it is either the heroin or 6-monoacetylmorphine analogue of methadol, and being a methadol it exhibits optical isomerism. The other methadols (acetylmethadol, methadol &c) have at least four optical isomers (see Orlaam).
See also[edit]
- Acetylmethadol
- Dimepheptanol (methadol)
References[edit]
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ Macdonald F (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. p. 1447. ISBN 978-0-412-46630-4. Retrieved 11 May 2012.
- ^ a b Gruber CM, Baptisti A (1963). "Estimating the acceptability of morphine and noracymethadol in postpartum patients". Clinical Pharmacology and Therapeutics. 4 (2): 172–81. doi:10.1002/cpt196342172. PMID 13950878. S2CID 19919842.
- ^ a b Lister RE (June 1966). "The toxicity of some of the newer narcotic analgesics". The Journal of Pharmacy and Pharmacology. 18 (6): 364–83. doi:10.1111/j.2042-7158.1966.tb07890.x. PMID 4381372. S2CID 32896981.
- ^ "Controlled Substances in Schedule I". Drug Enforcement Administration - Office of Diversion Control. Retrieved 2012-05-11.
- ^ Nordegren T (1 March 2002). The A-Z Encyclopedia of Alcohol and Drug Abuse. Universal-Publishers. p. 468. ISBN 978-1-58112-404-0. Retrieved 11 May 2012.