| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Hyponitrite | |||
| Systematic IUPAC name
Diazenebis(olate) | |||
| Other names
Hyponitrite(2–)
| |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| 3DMet | |||
| ChEBI | |||
| ChemSpider |
| ||
| 130273 | |||
| KEGG | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| N 2O2− 2 | |||
| Molar mass | 60.012 g·mol−1 | ||
| Conjugate acid | Hyponitrous acid | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
In chemistry, hyponitrite may refer to the anion N
2O2−
2 ([ON=NO]2−), or to any ionic compound that contains it. In organic chemistry, it may also refer to the group −O−N=N−O−, or any organic compound with the generic formula R1−O−N=N−O−R2, where R1 and R2 are organic groups.[1] Such compounds can be viewed as salts and esters of hyponitrous acid.
An acid hyponitrite is an ionic compound with the anion HN
2O−
2 ([HON=NO]−).
Hyponitrite ion[edit]
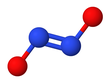
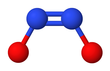
Hyponitrite exhibits cis–trans isomerism.[2]
The trans (E) form is generally found in hyponitrite salts such as sodium hyponitrite (Na
2N
2O
2) and silver(I) hyponitrite (Ag
2N
2O
2).
The cis (Z) form of sodium hyponitrite can be obtained too, but it is more reactive than the trans form.[2] The cis hyponitrite anion is nearly planar and almost symmetric, with lengths of about 140 pm for N−O bond and 120 pm for the N−N bond, and O−N−N angles of about 119°.[3]
Reactions[edit]
The hyponitrite ions can act as a bidentate ligand in either bridging or chelating mode. There is a bridging cis-hyponitrite group in the red dinuclear form of nitrosyl pentammine cobalt(III) chloride, [Co(NH3)5NO]Cl2.[4]
Hyponitrite can reduce elemental iodine to hydroiodic acid:[4]
- N
2O2−
2 + 3 I
2 + 3 H
2O → NO−
3 + NO−
2 + 6 HI
Hyponitrite esters[edit]
Organic trans-hyponitrites R1−O−N=N−O−R2 can be obtained by reacting trans silver(I) hyponitrite Ag
2N
2O
2 with various alkyl halides. For example, reaction with tert-butyl chloride yields trans di-tert-butyl hyponitrite.[5][6][7][8]
Other alkyl radicals reported in the literature include ethyl,[9] and benzyl.[10][11][12] These compounds can be a source of alkoxyl radicals.[13]
See also[edit]
Other nitrogen oxyanions include
- nitrate, NO−
3 - nitrite, NO−
2 - peroxonitrite, (peroxynitrite), OONO−
- peroxonitrate, HNO−
4 - trioxodinitrate, (hyponitrate), [ON=NO2]2−
- nitroxylate, [O2N−NO2]4−
- orthonitrate, NO3−
4
References[edit]
- ^ Hughes, M. N. (1968). "Hyponitrites". Quarterly Reviews, Chemical Society. 22: 1. doi:10.1039/QR9682200001.
- ^ a b Egon Wiberg, Arnold Frederick Holleman (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- ^ Feldmann, Claus; Jansen, Martin (1996). "Cis-Sodium Hyponitrite—A New Preparative Route and a Crystal Structure Analysis". Angewandte Chemie International Edition in English. 35 (15): 1728–1730. doi:10.1002/anie.199617281.
- ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Navamoney Arulsamy; D. Scott Bohle; Jerome A. Imonigie; Elizabeth S. Sagan (2000). "Correlation of the Product E/Z Framework Geometry and O/O vs O/N Regioselectivity in the Dialkylation of Hyponitrite". J. Am. Chem. Soc. 122 (23): 5539–5549. doi:10.1021/ja994261o.
- ^ Kiefer, Hansruedi; Traylor, T.G. (1966). "Di-t-butyl hyponitrite. A convenient source of t-butoxy radicals". Tetrahedron Letters. 7 (49): 6163–6168. doi:10.1016/s0040-4039(00)70159-6. ISSN 0040-4039.
- ^ Huang, R. L.; Lee, Tong-Wai; Ong, S. H. (1969). "Reactions of the α-methoxybenzyl radical in carbon tetrachloride and in other solvents. Carbon tetrachloride as a chlorinating agent". J. Chem. Soc. C (1): 40–44. doi:10.1039/j39690000040. ISSN 0022-4952.
- ^ Neuman, Robert C.; Bussey, Robert J. (1970). "High pressure studies. V. Activation volumes for combination and diffusion of geminate tert-butoxy radicals". Journal of the American Chemical Society. 92 (8): 2440–2445. doi:10.1021/ja00711a039. ISSN 0002-7863.
- ^ Partington, James R.; Shah, Chandulal C. (1932). "384. Hyponitrites. Part II : metallic salts. Part III : esters". Journal of the Chemical Society: 2589. doi:10.1039/jr9320002589. ISSN 0368-1769.
- ^ Ho, S. K.; de Sousa, J. B. (1961). "347. Alkoxy-radicals. Part I. The kinetics of thermal decomposition of dibenzyl hyponitrite in solution". Journal of the Chemical Society: 1788. doi:10.1039/jr9610001788. ISSN 0368-1769.
- ^ de SOUSA, J. B.; HO, S. K. (1960). "Disproportionation and Dimerization of the Benzyloxyl Free Radical in Solution". Nature. 186 (4727): 776–778. Bibcode:1960Natur.186..776D. doi:10.1038/186776a0. ISSN 0028-0836. S2CID 4248607.
- ^ Ray, N. H. (1960). "794. The rates of decomposition of free-radical polymerisation-catalysts: measurements of short half-lives by a thermal method". Journal of the Chemical Society: 4023. doi:10.1039/jr9600004023. ISSN 0368-1769.
- ^ Craig A. Ogle; Steven W. Martin; Michael P. Dziobak; Marek W. Urban; G. David Mendenhall (1983). "Decomposition rates, synthesis, and spectral properties of a series of alkyl hyponitrites". J. Org. Chem. 48 (21): 3728–3733. doi:10.1021/jo00169a023.