| |

| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
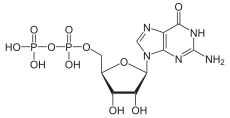
| C10H15N5O11P2 | |
| Molar mass | 443.200522 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Guanosine diphosphate, abbreviated GDP, is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside guanosine. GDP consists of a pyrophosphate group, a pentose sugar ribose, and the nucleobase guanine.[1]
GDP is the product of GTP dephosphorylation by GTPases, e.g., the G-proteins that are involved in signal transduction.
GDP is converted into GTP with the help of pyruvate kinase and phosphoenolpyruvate.
GDP and GTP[edit]
Hydrolysis of GTP into GDP[edit]
The hydrolysis of GTP to GDP is facilitated by GTPase enzymes, which utilize a conserved active site motif known as the GTPase-activating protein (GAP). Initially, a water molecule is coordinated by the active site residues of the GTPase enzyme. The water molecule attacks the γ-phosphate of GTP, leading to the formation of a pentavalent transition state. This transition state is stabilized by interactions with the active site residues, including conserved catalytic residues. As a result, the γ-phosphate is cleaved, and inorganic phosphate (Pi) is released. This step also causes a conformational change in the enzyme that promotes the release of GDP.[2]
Biochemical functions[edit]
Intracellular signaling[edit]
GDP is involved in intracellular signaling processes functioning as a critical regulator in the activity of GTPases. GTPases act as molecular switches, cycling between an active GTP-bound state and an inactive GDP-bound state. The interconversion between GDP and GTP is tightly controlled and serves as a molecular timer for signal transduction pathways. When an extracellular signal triggers the activation of a G-protein coupled receptor (GPCR), the associated G-protein exchanges its bound GDP for GTP, leading to a conformational change and activation of downstream signaling cascades.[3] This activation can stimulate a variety of cellular responses, including modulation of gene expression, cytoskeletal rearrangements, and regulation of enzymatic activities. The hydrolysis of GTP to GDP by the GTPase activity of the G-protein restoring the inactive state, thus terminates the signaling event.[4]
See also[edit]
References[edit]
- ^ Crane, Laura J; Miller, David Lee (1974). "Guanosine triphosphate and guanosine diphosphate as conformation-determining molecules. Differential interaction of a fluorescent probe with the guanosine nucleotide complexes of bacterial elongation factor Tu". Biochemistry. 13 (5): 933–939. doi:10.1021/bi00702a017. PMID 4591619.
- ^ Calixto, Ana R.; Moreira, Cátia; Pabis, Anna; Kötting, Carsten; Gerwert, Klaus; Rudack, Till; Kamerlin, Shina C.L. (2019-07-10). "GTP Hydrolysis Without an Active Site Base: A Unifying Mechanism for Ras and Related GTPases". Journal of the American Chemical Society. 141 (27): 10684–10701. doi:10.1021/jacs.9b03193. ISSN 0002-7863.
- ^ Downes, G. B.; Gautam, N. (1999-12-15). "The G protein subunit gene families". Genomics. 62 (3): 544–552. doi:10.1006/geno.1999.5992. ISSN 0888-7543. PMID 10644457.
- ^ Schmidt, Anja; Hall, Alan (2002-07-01). "Guanine nucleotide exchange factors for Rho GTPases: turning on the switch". Genes & Development. 16 (13): 1587–1609. doi:10.1101/gad.1003302. ISSN 0890-9369. PMID 12101119.