| |
| Names | |
|---|---|
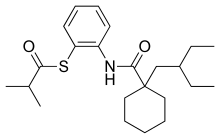
| IUPAC name
S-[2-({[1-(2-Ethylbutyl)cyclohexyl]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.250.741 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C23H35NO2S | |
| Molar mass | 389.5945 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dalcetrapib (INN,[1] codenamed JTT-705) is a CETP inhibitor which was being developed by Hoffmann–La Roche until May 2012.[2][3] The drug was aimed at raising the blood levels of HDL cholesterol.[4] Prevailing observations indicate that high HDL levels correlate with better overall cardiovascular health, though it remains unclear whether raising HDL levels consequently leads to an increase in cardiovascular health.[5]
A 24-week clinical trial showed that dalcetrapib did increase HDL-C levels, supporting the agent's desired effect.[6] Further, the dal-PLAQUE phase IIb trial found evidence of plaque reduction.[7] Plaque reduction is an anticipated observation following an increase in HDL.[citation needed]
As of 2010[update] five phase II trials had started and there was no evidence of the raised blood pressure seen with torcetrapib.[6]
dal-VESSEL phase IIb trial found no evidence of flow-mediated dilatation improvement. A 17% increase of Lp-PLA2 mass level was noted.[8] Lp-PLA2 is associated with coronary heart disease and stroke.[citation needed]
dal-OUTCOMES phase III trial passed its first interim review in July, 2011,[9] however, development was halted on May 7, 2012 “due to a lack of clinically meaningful efficacy.”.[3]
The results of dal-OUTCOMES III were published in November, 2012.[10]
A pharmacogenomic genome-wide association study (GWAS) reported that patients from the dal-OUTCOMES study bearing a protective allele at SNP rs1967309 in the ADCY9 gene may have benefited from dalcetrapib therapy.[11] Changes in inflammation and cholesterol efflux capacity may in part explain the benefits associated with the protective genotype.[12] The Dal-GenE trial was performed to validate these observations. This clinical trial is a randomized placebo-controlled study to evaluate the effects of dalcetrapib on cardiovascular risk in patients with recent acute coronary syndrome bearing the protective genotype.[13] The results published in 2022 showed that Dalcetrapib did not significantly reduce the risk of occurrence of the primary endpoint of ischaemic cardiovascular events at end of study in patients with an acute coronary syndrome within 1-3 months and the AA genotype at variant rs1967309 in the ADCY9 gene.[14]
See also[edit]
References[edit]
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 58" (PDF). World Health Organization. pp. 250–1. Retrieved 3 January 2017.
- ^ Huang Z; Inazu A; Nohara A; Higashikata T; Mabuchi H (December 2002). "Cholesteryl ester transfer protein inhibitor (JTT-705) and the development of atherosclerosis in rabbits with severe hypercholesterolaemia". Clin. Sci. 103 (6): 587–594. doi:10.1042/cs1030587. hdl:2297/15762. PMID 12444911. S2CID 22400248.
- ^ a b Simeon Bennett & Naomi Kresge. "Roche Drops After Halting Cholesterol Drug Development". Bloomberg.
- ^ Michelle Fay Cortez (November 5, 2012), "Roche's Good Cholesterol Drug Shows Negative Side Effects", Bloomberg Businessweek, archived from the original on November 8, 2012, retrieved November 6, 2012
- ^ "NIH stops clinical trial on combination cholesterol treatment". National Institute of Health. NHLBI. Retrieved June 2, 2011.
- ^ a b Stein; et al. (2010). "Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial". Eur. Heart J. 31 (4): 480–4888. doi:10.1093/eurheartj/ehp601. PMC 2821630. PMID 20097702.
- ^ Zahi A Fayad; et al. (2011). "Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial". The Lancet. 378 (9802): 1547–1559. doi:10.1016/S0140-6736(11)61383-4. PMC 4151875. PMID 21908036.
- ^ Thomas F. Lüscher; et al. (2012). "Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial". Eur. Heart J. 33 (7): 857–865. doi:10.1093/eurheartj/ehs019. PMC 3345558. PMID 22345126.
- ^ Gail Parziale. "Dalcetrapib and Anacetrapib: a Tale of Two CETPs". Archived from the original on 2011-12-19.
- ^ Schwartz, G. G.; Olsson, A. G.; Abt, M.; Ballantyne, C. M.; Barter, P. J.; Brumm, J.; Chaitman, B. R.; Holme, I. M.; Kallend, D.; Leiter, L. A.; Leitersdorf, E.; McMurray, J. J. V.; Mundl, H.; Nicholls, S. J.; Shah, P. K.; Tardif, J. C.; Wright, R. S.; Dal-Outcomes, I. (2012). "Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome" (PDF). New England Journal of Medicine. 367 (22): 2089–2099. doi:10.1056/NEJMoa1206797. PMID 23126252.
- ^ Tardif, Jean-Claude; Rhéaume, Eric; Lemieux Perreault, Louis-Philippe; Grégoire, Jean C.; Feroz Zada, Yassamin; Asselin, Géraldine; Provost, Sylvie; Barhdadi, Amina; Rhainds, David (2015-04-01). "Pharmacogenomic determinants of the cardiovascular effects of dalcetrapib". Circulation: Cardiovascular Genetics. 8 (2): 372–382. doi:10.1161/CIRCGENETICS.114.000663. ISSN 1942-3268. PMID 25583994.
- ^ Tardif, Jean-Claude; Rhainds, David; Brodeur, Mathieu; Feroz Zada, Yassamin; Fouodjio, René; Provost, Sylvie; Boulé, Marie; Alem, Sonia; Grégoire, Jean C. (2016-08-01). "Genotype-Dependent Effects of Dalcetrapib on Cholesterol Efflux and Inflammation: Concordance With Clinical Outcomes". Circulation: Cardiovascular Genetics. 9 (4): 340–348. doi:10.1161/CIRCGENETICS.116.001405. ISSN 1942-3268. PMC 4982759. PMID 27418594.
- ^ "Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 2016-12-02.
- ^ Tardif, Jean Claude; Pfeffer, Marc A; Kouz, Simon; Koenig, Wolfgang; Maggioni, Aldo P; McMurray, John J V; Mooser, Vincent; Waters, David D; Grégoire, Jean C; L’Allier, Philippe L; Wouter Jukema, J; White, Harvey D.; Heinonen, Therese; Black, Donald M; Laghrissi-Thode, Fouzia; Levesque, Sylvie; Guertin, Marie Claude; Dubé, Marie Pierre (14 October 2022). "Pharmacogenetics-guided dalcetrapib therapy after an acute coronary syndrome: the dal-GenE trial". European Heart Journal. 43 (39): 3947–3956. doi:10.1093/eurheartj/ehac374. hdl:1887/3567823.