| |
| Names | |
|---|---|
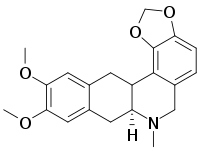
| Preferred IUPAC name
(7aS)-10,11-Dimethoxy-7-methyl-6,7,7a,8-tetrahydro-2H,5H-benzo[g][1,3]benzodioxolo[6,5,4-de]quinoline | |
| Other names
(S)-(+)-Dicentrine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H21NO4 | |
| Molar mass | 339.391 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dicentrine is an aporphinic alkaloid found in several plant species, mainly from family Lauraceae, including Lindera megaphylla.[1] At high doses, dicentrine shows antinociceptive activity in a mouse model of pain.[2] It probably acts via a TRPA1-dependent mechanism.[2]
References[edit]
- ^ Huang, Ray-Ling; Chen, Chien-Chih; Huang, Yu-Lin; Ou, Jun-Chih; Hu, Cheng-Po; Chen, Chieh-Fu; Chang, Chungming (1998). "Anti-Tumor Effects of d-Dicentrine from the Root of Lindera megaphylla". Planta Medica. 64 (3): 212–215. doi:10.1055/s-2006-957411. PMID 9581516.
- ^ a b Montrucchio, Deise Prehs; Córdova, Marina Machado; Santos, Adair Roberto Soares (2013-07-04). "Plant Derived Aporphinic Alkaloid S-(+)-Dicentrine Induces Antinociceptive Effect in Both Acute and Chronic Inflammatory Pain Models: Evidence for a Role of TRPA1 Channels". PLOS ONE. 8 (7): e67730. Bibcode:2013PLoSO...867730M. doi:10.1371/journal.pone.0067730. ISSN 1932-6203. PMC 3701576. PMID 23861794.