| |
| Names | |
|---|---|
| Preferred IUPAC name
3-Sulfanylpropanenitrile | |
| Other names
β-Mercaptopropionitrile, 2-Cyanoethanethiol, 3-Mercaptopropanenitrile
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.012.438 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H5NS | |
| Molar mass | 87.14 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.0696 g/cm3 |
| Boiling point | 30–32 °C (86–90 °F; 303–305 K) 0.08-0.12 mm |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P270, P271, P280, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P312, P321, P322, P330, P332+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
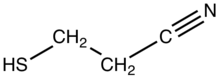
3-Mercaptopropionitrile is the organosulfur compound with the formula HSCH2CH2CN.[1] Containing both thiol and nitrile functional groups, it is a bifunctional compound. A colorless liquid, the compound has found some use as a masked form of thiolate.
Preparation and reactions[edit]
it is typically prepared from 3-chloropropionitrile via initial reaction with thiourea. The resulting isothiouronium salt hydrolyzes to the thiol.[2] A A variation on this preparation involves isolation of the disulfide (SCH2CH2CN)2. Zinc reduction of this disulfide gives the thiol.[3]
Thioethers derived from S-alkylation of 3-mercaptopropionitrile react with strong bases to give thiolate and acrylonitrile:
- RSCH2CH2CN + KOBu-t → RSK + CH2=CHCN + HOBu-t
The conversion illustrates the retro-Michael reaction. The thiolate is then hydrolyzed
- RSK + H+ → RSH + K+
References[edit]
- ^ Encyclopedia of Reagents for Organic Synthesis (1 ed.). Wiley. 2001-04-15. doi:10.1002/047084289x.rn02347. ISBN 978-0-471-93623-7. S2CID 242662674.
- ^ R. Eric Gerber; Carlos Hasbun; Larisa G. Dubenko; Mei Fong King; Donald E. Bierer (2000). "β-Mercaptopropionitrile". Organic Syntheses. 77: 186. doi:10.15227/orgsyn.077.0186.
- ^ Klose, J.; Reese, C. B.; Song, Q. (1997). "Preparation of 2-(2-cyanoethyl)sulfanyl-1H-isoindole-1,3-(2H)-dione and related sulfur-transfer agents". Tetrahedron. 53 (42): 14411–14416. doi:10.1016/S0040-4020(97)00924-1.